Illustration 1.
CH3(CH2)2CH(CH3)CH2CH3
When viewing a condensed formula of this kind, one must recognize that parentheses are used both to identify repeating units, such as the two methylene groups on the left side, and substituents, such as the methyl group on the right side. This formula is elaborated and named as follows:
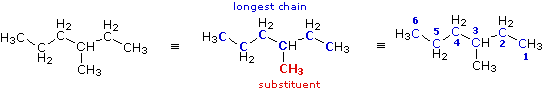 |
The condensed formula is expanded on the left. By inspection, the longest chain is seen to consist of six carbons, so the root name of this compound will be hexane. A single methyl substituent (colored red) is present, so this compound is a methylhexane. The location of the methyl group must be specified, since there are two possible isomers of this kind. Note that if the methyl group were located at the end of the chain, the longest chain would have seven carbons and the root name would be heptane not hexane. To locate the substituent the hexane chain must be numbered consecutively, starting from the end nearest a substituent. In this case it is the right end of the chain, and the methyl group is located on carbon #3. The IUPAC name is thus: 3-methylhexane .
Illustration 2.
(CH3)2C(C2H5)2
Again, the condensed formula is expanded on the left, the longest chain is identified (five carbons) and substituents are located and named. Because of the symmetrical substitution pattern, it does not matter at which end of the chain the numbering begins.
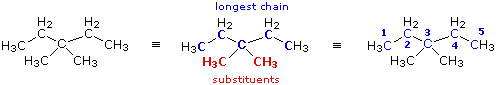 |
When two or more identical substituents are present in a molecule, a numerical prefix (di, tri, tetra etc.) is used to designate their number. However, each substituent must be given an identifying location number. Thus, the above compound is correctly named: 3,3-dimethylpentane.
Note that the isomer (CH3)2CHCH2CH(CH3)2 would be named 2,4-dimethylpentane.
Note that the isomer (CH3)2CHCH2CH(CH3)2 would be named 2,4-dimethylpentane.
Illustration 3.
(CH3)2CHCH2CH(C2H5)C(CH3)3
This example illustrates some sub-rules of the IUPAC system that must be used in complex cases. The expanded and line formulas are shown below.
Sub-Rules for IUPAC Nomenclature
1. If there are two or more longest chains of equal length, the one having the largest number of substituents is chosen.
2. If both ends of the root chain have equidistant substituents: (i) begin numbering at the end nearest a third substituent, if one is present. (ii) begin numbering at the end nearest the first cited group (alphabetical order). |
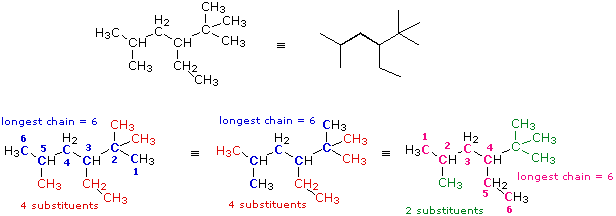
In this case several six-carbon chains can be identified. Some (colored blue) are identical in that they have the same number, kind and location of substituents. The IUPAC name derived from these chains will not change. Some (colored magenta) differ in the number, kind and location of substituents, and will result in a different name. From rule 1 above the blue chain is chosen, and it will be numbered from the right hand end by application of rule (i). Remembering the alphabetical priority, we assign the following IUPAC name: 3-ethyl-2,2,5-trimethylhexane.
Illustration 4.
The following are additional examples of more complex structures and their names.
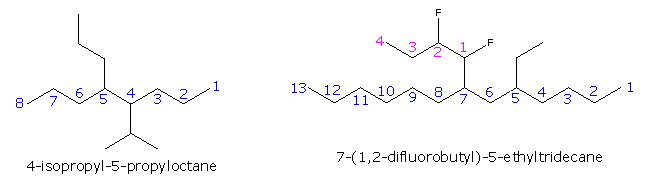 |
The formula on the right shows how a complex substituent may be given a supplementary numbering. In such cases the full substituent name is displayed within parenthesis and is alphabetized including numerical prefixes such as 'di'.
Illustration 5.
Write a structural formula for the compound 3,4-dichloro-4-ethyl-5-methylheptane.
First, we draw a chain of seven carbon atoms to represent the root name "heptane". This chain can be numbered from either end, since no substituents are yet attached. From the IUPAC name we know there are two chlorine, one ethyl and one methyl substituents. The numbers tell us where the substituents are located on the chain, so they can be attached, as shown in the middle structure below. Finally, hydrogen atoms are introduced to satisfy the tetravalency of carbon. The structural formula on the right can then be written in condensed form
First, we draw a chain of seven carbon atoms to represent the root name "heptane". This chain can be numbered from either end, since no substituents are yet attached. From the IUPAC name we know there are two chlorine, one ethyl and one methyl substituents. The numbers tell us where the substituents are located on the chain, so they can be attached, as shown in the middle structure below. Finally, hydrogen atoms are introduced to satisfy the tetravalency of carbon. The structural formula on the right can then be written in condensed form
as:
CH3CH2CHClCCl(C2H5)CH(CH3)CH2CH3 orC2H5CHClCCl(C2H5)CH(CH3)C2H5
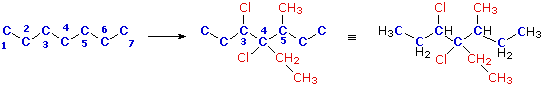 |
In naming this compound it should be noted that the seven carbon chain is numbered from the end nearest the chlorine by applying rule (ii) above.
Examples of the IUPAC Rules in Practice
Illustration 1 (CH3)2C=CHCH2C(CH3)3
|
Illustration 2 (CH3CH2CH2)2C=CH2
|
|---|
Expanding these formulas we have:
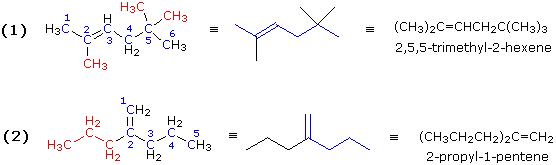
Illustration 3 (C2H5)2C=CHCH(CH3)2
|
Illustration 4 CH2=C(CH3)CH(CH3)C(C2H5)=CH2
|
|---|
The next two examples illustrate additional features of chain numbering. As customary, the root chain is colored blue and substituents are red.
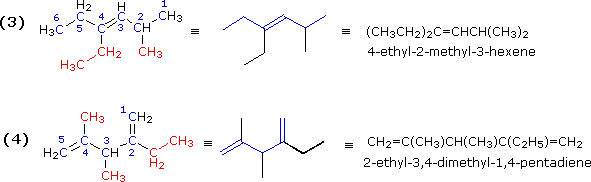
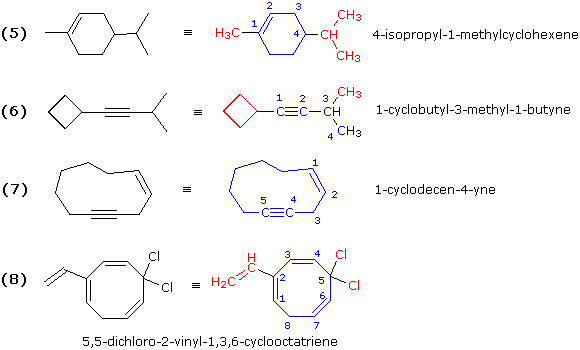
Illustrations 5, 6, 7 & 8
Other Functional Groups
The previous discussion has focused on the carbon framework that characterizes organic compounds, and has provided a set of nomenclature rules that, with some modification, apply to all such compounds. An introduction to functional group nomenclature was limited to carbon-carbon double and triple bonds, as well as simple halogen groups. There are, however, many other functional groups that are covered by the IUPAC nomenclature system. A summary of some of these groups and the characteristic nomenclature terms for each is presented in the following table. Specific examples of their nomenclature will be provided as the chemistry of each group is discussed.
Group Names and Suffixes for Some Common Functional Groups
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note that only one functional group suffix, other than "ene" and "yne", may be used in a given name. The following table gives the priority order of suffix carrying groups in arriving at a IUPAC name. When a compound contains more than one kind of group in this list, the principal characteristic group is the one nearest the top. All other groups are then cited as prefixes.
Decreasing Priority Order of Principle Characteristic Groups Identified by a Suffix
| 1. Acids (in the order COOH, C(O)O2H; then their S and Se derivatives, followed by sulfonic, sulfinic, selenonic, etc., phosphonic, arsonic, etc., acids) 2. Anhydrides 3. Esters 4. Acid halides 5. Amides 6. Hydrazides 7. Imides 8. Nitriles 9. Aldehydes, followed by thioaldehydes & selenoaldehydes 10. Ketones, followed by thioketones & selenoketones 11. Alcohols and Phenols, followed by thiols & selenols 12. Hydroperoxides, followed by thiohydroperoxides & selenohydroperoxides 13. Amines 14. Imines 15. Hydrazines, Phosphanes, etc. 16. Ethers followed by sulfides & selenides 17. Peroxides, followed by disulfides & diselenides |