Tuesday 4 February 2014
Sunday 2 February 2014
electrophillic aromatic substitution and diazo coupling
Azo coupling is the most widely used industrial reaction in the production of dyes, lakes and pigments.
Aromatic diazonium ions acts as electrophiles in coupling reactions with activated aromatics such as anilines or phenols.
The substitution normally occurs at the para position, except when this position is already occupied, in which case ortho position is favoured.
The pH of solution is quite important; it must be mildly acidic or neutral, since no reaction takes place if the pH is too low.Let us take an example of methyl orange.
Due to their
positive charge, diazonium cations, which are generated by treatment of
aromatic amines with nitrous acid and a stronger mineral acid, may
participate in an electrophilic
aromatic substitution as an
electrophile.
The electrophilic reaction center is the terminal nitrogen of the
-N=N+ group. As a result, two aromatic compounds are coupled by a -N=N-
group. This is known as the azo group (diazo
group). The corresponding reaction is called diazonium
coupling (diazo coupling, azo coupling). However, the
electrophilicity of diazonium ions is only relatively weak, as their positive
charge is delocalized. The unsubstituted benzenediazonium cation may react only
with strongly activated aromatic compounds, such as phenolates and amines.
By introducing
electron-withdrawing substituents in ortho
or para position regarding the azo group,
the diazonium ions' electrophilicity may be increased to such a degree that
diazonium coupling also occurs with phenols and phenolic ethers, such as
anisole.
Due to the
fact that diazonium cations are poor electrophiles and relatively bulky
species, mainly para substitution usually takes place in
diazonium coupling. In the case of para substitution, steric
hindrance is at its weakest, while the positive charge's stabilization is at
its largest, in the σ complex (and, thus, in the transition state). If
the para position is already occupied by another
substituent, ortho substitution occurs.
methyl orange is used in acid base titrations;
| Methyl orange Methyl orange is one of the indicators commonly used in titrations. In an alkaline solution, methyl orange is yellow and the structure is: 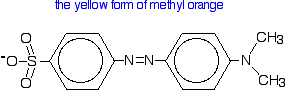 In fact, the hydrogen ion attaches to one of the nitrogens in the nitrogen-nitrogen double bond to give a structure which might be drawn like this: 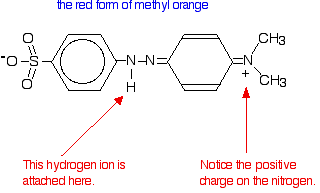 | |
Note: You may find other structures for this with different arrangements of the bonds (although always with the hydrogen attached to that same nitrogen). The truth is that there is delocalisation over the entire structure, and no simple picture will show it properly. Don't worry about this exact structure - it is just to show a real case where the colour of a compound is drastically changed by the presence or absence of a hydrogen ion. | |
| You have the same sort of equilibrium between the two forms of methyl orange as in the litmus case - but the colours are different. | |
Subscribe to:
Posts (Atom)













