Wednesday, 14 January 2015
Saturday, 10 January 2015
Aldol-like Reactions Michael reaction
Aldol-like Reactions
Michael reaction
Aldol condensations and aldol-like condensations yield α,β-unsaturated carbonyl compounds.
As they enable a nucleophilic attack on the polarized double bond, α,β-unsaturated carbonyl compounds are critical starting products in many syntheses.
A nucleophilic attack on the double bond may be possible because of the double bond's polarization by the carbonyl group and the resonance stabilization of the α carbanion, which is generated by a nucleophilic attack on the β carbon, by conjugation with the carbonyl group.
The intermediate α carbanion is actually an enolate that is subsequently protonated to the enol. The enol is in equilibrium with the ketone or aldehyde through keto-enol tautomerism.
All in all, the reaction is a 1,4-addition to an α,β-unsaturated carbonyl compound. It is called Michael reaction (after Arthur Michael).
The original, classical Michael reaction is the addition of an enolate to an α,β-unsaturated carbonyl compound. In this case, the product of a Michael reaction is a 1,5-dicarbonyl compound. However, a variety of further nucleophiles may be applied.
If the nucleophile's nucleophilicity is low, the Michael reaction may occasionally be made possible through acid catalysis.
Theoretically, the Michael acceptor (the α,β-unsaturated carbonyl compound) contains two electrophilic positions, the β carbon and the carbonyl carbon. However, the product of the 1,4-addition usually exceeds the product of the 1,2-addition, as ...
- ... Michael reaction is reversible, and, thus, the thermodynamically more stable product, which contains the strong carbon-oxygen double bond, is favored.
- ... due to the Michael reaction's reversibility, the less stable 1,2-addition product is reconverted into the starting products and consequently into the more stable 1,4-addition product.
If extremely strong nucleophiles, such as hydrides or an alkyllithium compound, are applied to the Michael reaction, the reversibility is restricted. The reaction is then kinetically controlled. As a result, the 1,2-addition exceeds the 1,4-addition.
Aldol-like Reactions
Many variations of aldol reaction were established. These work on the same principle as classical aldol reaction does, such as ...
- ... intramolecular aldol reaction, which yields cyclic compounds.
- ... crossed aldol reaction, in which two different carbonyl compounds are applied together, resulting in a variety of products.
- ... Knoevenagel reaction, in which particularly strongly CH-acidic compounds, such as 1,3-dicarbonyl compounds or nitroalkanes, are applied instead of a "simple" enolate.
- ... Michael reaction, in which the conjugated base (anion) of a CH-acidic compound is added to the β carbon of an α,β-unsaturated carbonyl compound. If the CH-acidic compound is an aldehyde or a ketone, the Michael reaction yields a 1,5-dicarbonyl compound.
- ... Robinson annulation, which is a combination of a Michael reaction and a subsequent intramolecular aldol condensation. Robinson annulation is useful for the construction of complex aliphatic ring skeletons, such as that of steroids and terpenes.
Knoevenagel condensation:InKnoevenagelcondensation, particularly strongly CH-acidic compounds, such as 1,3-dicarbonyl compounds or nitroalkanes, are applied instead of a "simple" enolate.Knoevenagel Condensation
In a variation of the aldol reaction, other CH-acidic compounds are applied instead of the classical enol. Theoretically, every carbanion is capable of nucleophilically attacking the electrophilic carbonyl group of aldehydes and ketones.- KNOEVENAGEL CONDENSATION
- The condensation of aldehydes or ketones with a variety of CH-acidic compounds in the presence of a base, such as a tertiary amine or KOH, is called Knoevenagel condensation.
The reaction is named after Emil Knoevenagel (1865-1921). In comparison to aldehydes and ketones (pKa values: 14-20), 1,3-diketones or β-keto carbonyl compounds are much stronger acids (pKa values: 5-13). Thus, they can relatively easily be deprotonated to the corresponding carbanion or enolate. Nitroalkanes, depicted in the second example in the illustration below, are considerably CH-acidic compounds (pKa values about 10). As a result, they may be successfully applied in the Knoevenagel condensation. This reaction is also often called the Henry reaction. Due to the relatively high acidity of the CH-acidic compounds applied in the Knoevenagel condensation, only a relatively weak base, such as an amine or KOH, is required. Much like in aldol reactions, the addition of the carbanion to the carbonyl compound is frequently followed by dehydration.
Crossed Aldol Reactions
Crossed Aldol Reactions
In a crossed aldol reaction, two different carbonyl compounds are applied. If both carbonyl compounds contain an α hydrogen atom, both may act as electrophilic carbonyl compound, as well as nucleophilic enol or enolate. In addition, each enol may nucleophilically attack the two different carbonyl compounds. As a result, such a crossed aldol reaction yields four different products. Therefore, crossed aldol reactions of this kind are synthetically less valuable. The starting products depicted in the illustration are symmetric ketones. If this were not the case additional products would be possible, due to the problem of regioselectivity.
If a starting product does not contain any α hydrogen atom, the variety of possible products is reduced to two, as this starting product can only act as an electrophilic carbonyl compound, though it cannot act as a nucleophilic enolate. Benzaldehyde, which contains no α hydrogen atoms, is such an aldehyde.
If benzaldehyde is converted with acetone, for instance, two different products (aside from different stereoisomers) may principally be formed, as acetone may react with benzaldehyde (product "A+B") as well as another acetone molecule (product "A+A"). However, practically speaking, the reaction basically yields product "A+B", as the electrophilicity and, thus, the reactivity of the aldehyde are considerably higher than that of the ketone. The reaction is known as Claisen-Schmidtreaction (after Ludwig Claisen). If tert-butyl methyl ketone is applied in this reaction in place of acetone, only one product is usually obtained, as, due to strong steric interactions, tert-butyl methyl ketone virtually never reacts with any other tert-butyl methyl ketone molecule.
aldol condensation reaction and other condensation reactions
Aldol Reactions
This reaction also involves the attack of a nucleophile, but the nucleophile is a carbanion generated from an aldehyde or ketone.
This requires a base to abstract a proton from the carbonyl compound to generate the carbanion.
The carbanion so generated may now attack another molecule of aldehyde or ketone to generate a β-hydroxy carbonyl compound which may be dehydrated to generate an α,β-unsaturated carbonyl compound (Scheme 2).
The reaction is almost complete for aldehydes but in case of even simple ketones, the reaction almost completely lies to the left.
The reason for such an observation lies in the reversibility of the formation of the carbanion.
In such cases, where the attack of the carbanion the carbonyl compound is slower than the reprotonation of the carbonyl compound, the product will be formed only in small amounts.
The reaction can be made to proceed quantitatively, if one of the products is removed continuously thereby pulling the reaction to the right.
MECHANISM OF THE ALDOL CONDENSATION
| |
1. MECHANISM OF THE ALDOL REACTION
| |
Step 1: First, an acid-base reaction. Hydroxide functions as a base and removes the acidic α-hydrogen giving the reactive enolate. | 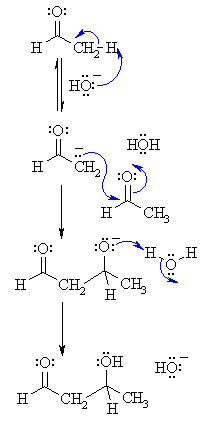 |
| Step 2: The nucleophilic enolate attacks the aldehyde at the electrophilic carbonyl C in a nucleophilic addition type process giving an intermediate alkoxide. | |
| Step 3: An acid-base reaction. The alkoxide deprotonates a water molecule creating hydroxide and the β-hydroxyaldehydes or aldol product. | |
2. MECHANISM OF THE DEHYDRATION OF THE ALDOL PRODUCT
| |
Step 1: First, an acid-base reaction. Hydroxide functions as a base and removes an acidic α-hydrogen giving the reactive enolate. | 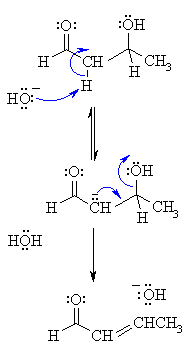 |

Scheme 2
The β-hydroxy compound so formed may undergo elimination in the presence of excess of base to generate an α,β-unsaturated carbonyl compound.
The reaction is evidently dependent on both the concentration of the base and the β-hydroxy carbonyl compound and is found to proceed by E1cB pathway.
It is to be noted that this reaction is essentially a reaction involving the enolate ions.
ALDOL CONDENSATION IN ACIDIC MEDIUM
Since enol formation from an aldehyde or ketone is feasible even in acid solution, so this reaction can be carried out using an acid as a catalyst.
The reaction now follows a completely different pathway and almost always leads to the formation of the dehydrated product.
Here, the carbonyl compound forms an equilibrium concentration of enol which then attacks the protonated form of the carbonyl compound to give the “aldol” which may undergo elimination by E1 pathway to give the α,β-unsaturated carbonyl compound (Scheme 3).

Scheme 3
A further complication may arise on subjecting unsymmetrical ketones to aldol reaction.
Now, there are two potential enols/enolates that could be formed and hence a mixture of products will be obtained.
Some examples of such ketones are t-butyl ketones, acetophenone and its derivatives and lactones (Scheme 4).

Scheme 4
An aldol reaction may also be carried out between an aldehyde and a ketone or between two different aldehydes and ketones.
Such a reaction is called a cross aldol reaction.
Now in cases where both the coupling partners possess α-H are not of any synthetic value as four different products may be produced.
However, where one of the coupling partners is only capable of enolizing and is more electrophilic than the other partner, the aldol reaction may be fruitful in giving only one preferential product.
Thus, the Claisen-Schmidt condensation of aromatic aldehydes with aliphatic aldehydes or methyl ketones using a base may be considered as of this type (Scheme 5).

Scheme 5
Similarly the importance of high electrophilicity of the non enolizing partner can be understood from the following example.

The reaction proceeds via the usual base catalyzed pathway. In the first step, the enolizable partner undergoes enolization in the presence of the base.

Next, the enolate attacks the electrophile benzaldehyde to form an aldol which then dehydrates by E1cB pathway.

- Reagents : commonly a base such as NaOH or KOH is added to the aldehyde.
- The reaction involves an aldehyde enolate reacting with another molecule of the aldehyde.
- Remember enolates are good nucleophiles and carbonyl C are good electrophiles.
- Since the pKa of an aldehyde is close to that of NaOH, both enolate and aldehyde are present simultaneously.
- The products of these reactions are β-hydroxyaldehydes or aldehyde-alcohols = aldols.
- The simplest aldol reaction is the condensation of ethanal. This is shown below in 2 different representations (the line diagrams are less cluttered).....
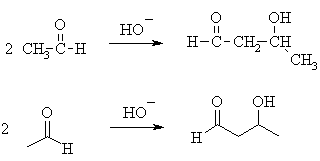 | ||
| Try to identify the enolate portion and the carbonyl portion in the different representations |
The enolate (1) formed in this step could attack another molecule of unenolized ketone but did not since ketones are less reactive than aldehydes.
Thus, the enolate chooses to attack the better electrophile (4-nitrobenzaldehyde).

Lithium enolates derived from carbonyl compounds in THF at low temperature by using lithium diisopropylamide (LDA) as base.
The reaction is kinetically controlled and therefore converted fast enough to prevent the enolate to react with a molecule of unenolized carbonyl compound.

Now, if a second carbonyl compound is added, then it also complexes with the lithium cation allowing the aldol reaction to take place from a six membered transition state.

The reaction works well even if the electrophilic partner is an enolizable aldehyde.
Thus, the lithium enolate of propiophenone can react with propan-1-al to form 3-hydroxy-2-methyl-1-phenylpentan-1-one selectively even when propan-1-al is an enolizable aldehydes (Scheme 6).
But it must be noted that the reaction is actually carried out in two steps. In the first step, lithium enolate of propiophenone is formed, then, the electrophilic partner is added.
- Dicarbonyl compounds can be used to give intramolecular Aldol reactions.
- This means the enolate component and the carbonyl component are parts of a single larger molecule.
- The selectivity is typically controlled by the stability of the cyclic Aldol product.
- This favours the formation of the more stable 5- or 6-membered rings (review)
- To work out the best product, check the permutations that are possible and look for the favourable ring sizes.
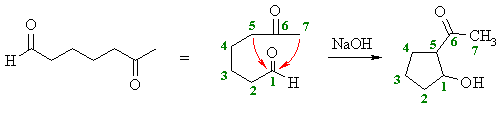
Intramolecular aldol reaction
Molecules which contain two carbonyl functionalities have the possibility of forming a ring through an intramolecular aldol reaction. In most cases two sets of α hydrogens need to be considered. As with most ring forming reaction five and six membered rings are preferred.
As with other aldol reaction the addition of heat causes an aldol condensation to occur.
Benzoin Condensation
A reaction similar in nature with the aldol reaction but occurs in the presence of a very concentration of base and with substrates lacking an α-H like benzaldehyde, formaldehyde etc. This reaction involves a hydride transfer from one molecule of aldehyde to other leading to an overall disproportionation where one molecule of aldehyde is oxidized to carboxylic acid where as the other is reduced to an alcohol (Scheme 9).

Scheme 9
Aromatic aldehydes in the presence of catalytic amount of cyanide as a nucleophile may undergobenzoin condensation where cyanide first attacks a molecule of aldehyde to form an intermediate. Next, an intramolecular hydride transfer takes place followed a carbanionic attack on another aldehyde molecule. The intermediate so formed undergoes elimination of cyanide to form α-hydroxyketone (Scheme 10).

Scheme 10
Cyanide is considered ideal for catalysing this reaction due to (1) its ability to act as a nucleophile, (2) its ability to act as a leaving group and (c) its ability through electron withdrawal to increase the acidity of the C-H bond and to stabilize the carbanion.
When benzaldehyde is used as reactant in the above reaction the product is called benzoin and hence the reaction has been named benzoin condensation. These α-hydroxyketones may be easily oxidized to the 1,2-diketones which under the action of hydroxylic base undergo rearrangement to form benzilic acid (Scheme 11).

Scheme 11
Subscribe to:
Posts (Atom)
















