Saturday 16 August 2014
Friday 15 August 2014
alkynes some reaction mechanisms
Alkynes- addition of hydrogen halides | |||||||||||||||||||||||||||||||||||
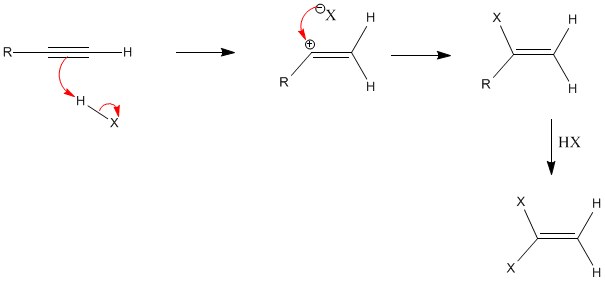
|
alkene -reaction summary
Alkenes- addition of Br2 | |||||||||||||||||||||||||||||||||||
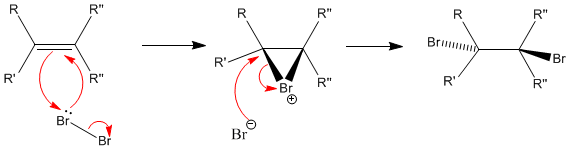
|
Alkenes- electrophilic addition of Br2 and H2O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
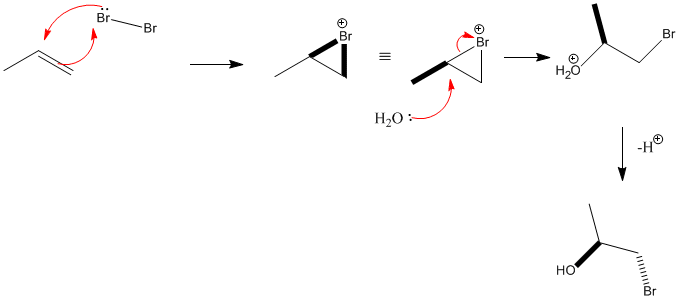
|
Subscribe to:
Posts (Atom)
Alkynes- addition of hydrogen halides | |||||||||||||||||||||||||||||||||||

|
Alkenes- addition of Br2 | |||||||||||||||||||||||||||||||||||

|
Alkenes- electrophilic addition of Br2 and H2O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

|