Periods and Blocks
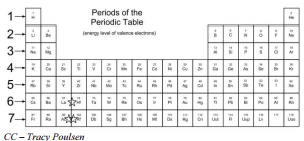
There are seven horizontal rows of the periodic table, called periods. The length of each period is determined by the number of electrons that are capable of occupying the sublevels that fill during that period, as seen in the table below.
Recall that the four different sublevels each consist of a different number of orbitals. The sublevel has one orbital, the sublevel has three orbitals, the sublevel has five orbitals, and the sublevel has seven orbitals. In the first period, only the sublevel is being filled. Since all orbitals can hold two electrons, the entire first period consists of just two elements. In the second period, the sublevel, with two electrons, and the sublevel, with six electrons, are being filled. Consequently, the second period contains eight elements. The third period is similar to the second, filling the and sublevels. Notice that the sublevel does not actually fill until after the sublevel. This results in the fourth period containing 18 elements due to the additional 10 electrons that are contributed by the sublevel. The fifth period is similar to the fourth. After the sublevel fills, the sublevel with its 14 electrons fills. This is followed by the and the . The total number of elements in the sixth period is 32. The later elements in the seventh period are still being created. So while there are a possible of 32 elements in the period, the current number is slightly less.
The period to which a given element belongs can easily be determined from its electron configuration. For example, consider the element nickel . Its electron configuration is . The highest occupied principal energy level is the fourth, indicated by the 4 in the portion of the configuration. Therefore, nickel can be found in the fourth period of the periodic table.
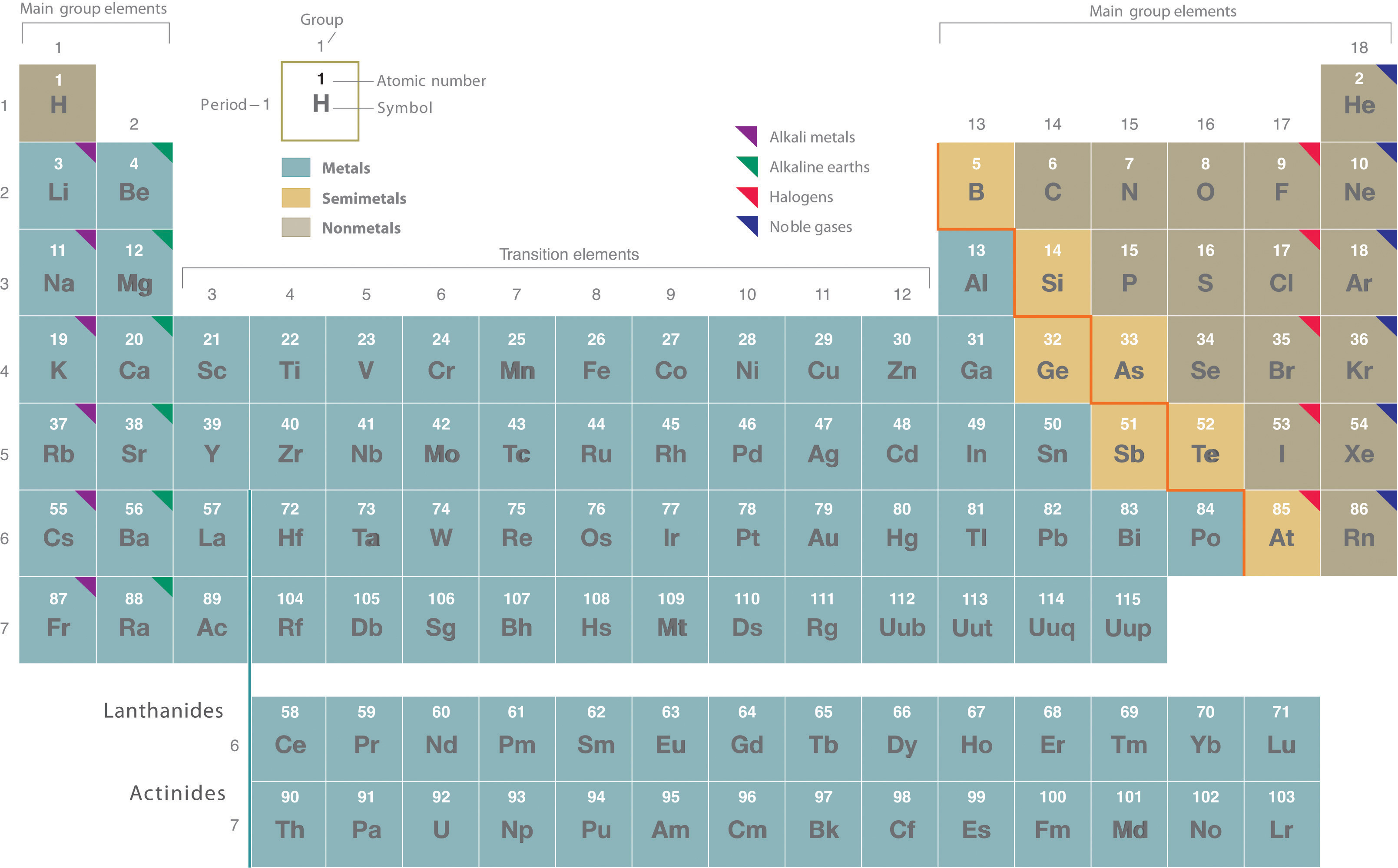
Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. The , , , and blocks are illustrated below.
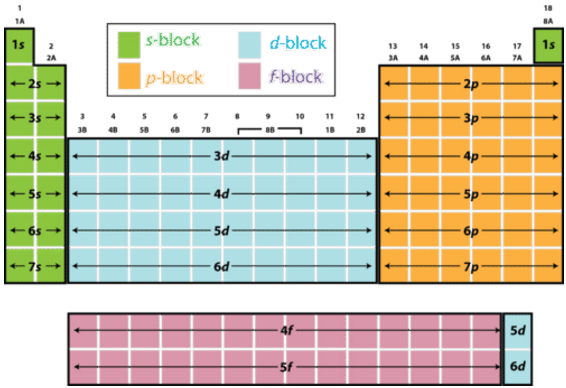
Figure 6.8.1: The periodic table labeled with sublevel blocks.
The figure also illustrates how the sublevel is always one principal level behind the period in which that sublevel occurs. In other words, the sublevel fills during the fourth period. The sublevel is always two levels behind. The sublevel belongs to the sixth period
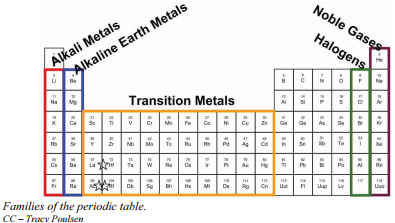
Families of the Periodic Table
Remember that Mendeleev arranged the periodic table so that elements with the most similar properties were placed in the same group. A group is a vertical column of the periodic table. All of the 1A elements have one valence electron. This is what causes these elements to react in the same ways as the other members of the family. The elements in 1A are all very reactive and form compounds in the same ratios with similar properties with other elements. Because of their similarities in their chemical properties, Mendeleev put these elements into the same group. Group 1A is also known as the alkali metals. Although most metals tend to be very hard, these metals are actually soft and can be easily cut.
Group 2A is also called the alkaline earth metals. Once again, because of their similarities in electron configurations, these elements have similar properties to each other. The same pattern is true of other groups on the periodic table. Remember, Mendeleev arranged the table so that elements with the most similar properties were in the same group on the periodic table.
It is important to recognize a couple of other important groups on the periodic table by their group name. Group 7A (or 17) elements are also called halogens. This group contains very reactive nonmetal elements.
The noble gases are in group 8A. These elements also have similar properties to each other, the most significant property being that they are extremely unreactive, rarely forming compounds. We will learn the reason for this later, when we discuss how compounds form. The elements in this group are also gases at room temperature.
An alternate numbering system numbers all of the , , and block elements from 1-18. In this numbering system, group 1A is group 1; group 2A is group 2; the halogens (7A) are group 17; and the noble gases (8A) are group 18. You will come across periodic tables with both numbering systems. It is important to recognize which numbering system is being used and to be able to find the number of valence electrons in the main block elements regardless of which numbering system is being used.
Periods of the Periodic Table
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group numbera | [hide]1 | 2 | 3d | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mendeleev (I–VIII) | I | II | III | IV | V | VI | VII | VIII | I | II | III | IV | V | VI | VII | b | ||||
| CAS (US, pattern A-B-A) | IA | IIA | IIIB | IVB | VB | VIB | VIIB | VIIIB | IB | IIB | IIIA | IVA | VA | VIA | VIIA | VIIIA | ||||
| old IUPAC (Europe, pattern A-B) | IA | IIA | IIIA | IVA | VA | VIA | VIIA | VIII | IB | IIB | IIIB | IVB | VB | VIB | VIIB | 0 | ||||
| Trivial name | Alkali metals | Alkaline earth metals | Coinage metalse | Volatile metalse | Icosagense | Crystallogense | Pnictogens | Chalcogens | Halogens | Noble gases | ||||||||||
| Name by element | Lithium group | Beryllium group | Scandium group | Titanium group | Vanadium group | Chromium group | Manganese group | Iron group | Cobalt group | Nickel group | Copper group | Zinc group | Boron group | Carbon group | Nitrogen group | Oxygen group | Fluorine group | Helium orNeon group | ||
| Period 1 | Hc | He | ||||||||||||||||||
| Period 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||
| Period 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||
| Period 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||
| Period 5 | Rb | Sr | d | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Period 6 | Cs | Ba | La–Yb | Lud | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |
| Period 7 | Fr | Ra | Ac–No | Lrd | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |
| a Current, modern IUPAC group number. | ||||||||||||||||||||
| b The noble gases had not yet been discovered at the time of Mendeleev's original table. Later (1902), Mendeleev accepted the evidence for the existence of the noble gases, and placed them in a separate "group 0". | ||||||||||||||||||||
| c Hydrogen (H), while placed in column 1, is not considered to be in the group alkali metals. | ||||||||||||||||||||
| d Group 3: Depending on the source, this group includes lutetium (Lu) and lawrencium (Lr), or lanthanum (La) and actinium (Ac), or the whole set of 15 lanthanides and 15 actinides, as the entries below scandium and yttrium. | ||||||||||||||||||||
| e This group name is not recommended by IUPAC. | ||||||||||||||||||||