Amines are derivatives of ammonia in which one or more of the hydrogens has been replaced by an alkyl or aryl group. The nomenclature of amines is complicated by the fact that several different nomenclature systems exist, and there is no clear preference for one over the others. Furthermore, the terms primary (1º), secondary (2º) & tertiary (3º) are used to classify amines in a completely different manner than they were used for alcohols or alkyl halides. When applied to amines these terms refer to the number of alkyl (or aryl) substituents bonded to the nitrogen atom, whereas in other cases they refer to the nature of an alkyl group. The four compounds shown in the top row of the following diagram are all C4H11N isomers. The first two are classified as 1º-amines, since only one alkyl group is bonded to the nitrogen; however, the alkyl group is primary in the first example and tertiary in the second. The third and fourth
compounds in the row are 2º and 3º-amines respectively. A nitrogen bonded to four alkyl groups will necessarily be positively charged, and is called a 4º-ammonium cation. Forexample, (CH3)4N(+) Br(–) is tetramethylammonium bromide.
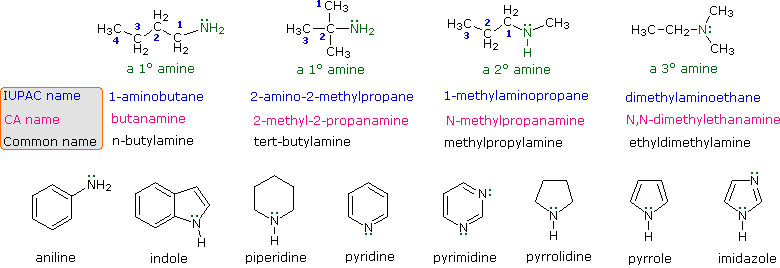
compounds in the row are 2º and 3º-amines respectively. A nitrogen bonded to four alkyl groups will necessarily be positively charged, and is called a 4º-ammonium cation. Forexample, (CH3)4N(+) Br(–) is tetramethylammonium bromide.


No comments:
Post a Comment