Estimation of Carbon and Hydrogen
Carbon and hydrogen present in an organic compound are estimated by 'Liebig's method'.
These elements are estimated by its combustion in an atmosphere of pure oxygen to form carbon dioxide (CO2) and water (H2O).
The apparatus used for the estimation of carbon and hydrogen consists of a long hard-glass combustion tube packed with copper oxide and coarse copper oxide. Left end of the combustion tube is connected to the oxygen supply, while at the other end, two pre-weighed absorption tubes are placed. One of the absorption tube is packed with magnesium perchlorate which absorbs water vapour, and the other is packed with KOH or asbestos which absorbs CO2.
Fig:16.15 Apparatus for the estimating carbon and hydrogen
Procedure
A small quantity (0.2 to 0.3 g) of the organic compound is placed in a porcelain / platinum boat. The boat is placed in the combustion tube and is heated at 400o to 450oC. Pure dry oxygen is slowly passed through the tube. Combustion is complete in 8 to 10 hours. It results in the formation of CO2 and H2O from the carbon and hydrogen in the compound. They are absorbed in the absorption tubes at the end.
The absorption tubes are removed and weighed to determine the amount of CO2 and H2O formed in the reaction. The masses and the percentages of C and H in the compound are calculated as follows.
Let,
The mass of the organic compound taken be = W g
Increase in the mass of magnesium perchlorate tube = W1 g
Increase in the mass of the KOH tube = W2 g
So,
Mass of water formed = W1 g
Mass of carbon dioxide formed = W2 g
Nitrogen can be estimated by either of the two methods:
- Dumas' method
- Kjeldahl's method
Dumas' method
A known mass of the organic compound is heated with cupric oxide in an atmosphere of carbon dioxide. The carbon and hydrogen in the compound are oxidised to carbon dioxide and water respectively, while nitrogen is set free. Any oxide of nitrogen produced during this process, is reduced back to free nitrogen by heated copper gauze. The gaseous mixture consisting of CO2, H2O and N2 is collected over an aqueous solution of potassium hydroxide. All the gases except nitrogen are absorbed by the solution. The volume of gas (nitrogen) collected is measured. From the volume of nitrogen obtained the percentage of nitrogen in the compound is calculated.
Fig:16.16 Apparatus for the estimation of nitrogen by Dumas' method
Calculations
Let,
The mass of the organic compound taken be = W g
Volume of nitrogen collected = V1 g
Atmospheric pressure = P mm Hg
Temperature at which gas is collected = T1 K
Therefore,
Pressure of the N2 gas, P1 = (P - p) mm of Hg

 (1 mol of N2 = 28 g = 22400 mL)
(1 mol of N2 = 28 g = 22400 mL)Kjeldahl's method
Kjeldahl's method is a faster method than Dumas' method. However, this method is used only for those organic compounds that are converted quantitatively to ammonium sulphate on heating strongly with concentrated sulphuric acid.
Kjeldahl's method cannot be used for the organic compounds,- Containing nitrogen in the ring, e.g., pyridine, quinoline etc.
- Containing nitro (-NO2) and diazo (-N = N-) groups.
Kjeldahl's method involves two steps:
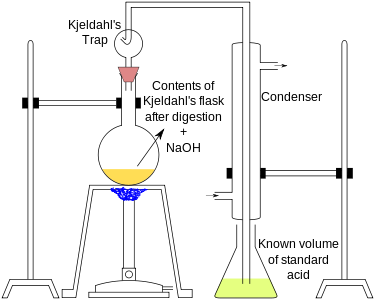
A known mass (0.3 to 0.5 g) of the given organic compound is digested with concentrated H2SO4, in presence of a small quantity of potassium sulphate and copper sulphate in a Kjeldahl's flask. Potassium sulphate raises the boiling point of sulphuric acid and copper sulphate catalyzes the digestion. In 3 to 4 hours, the organic compound is completely decomposed to form ammonium sulphate.
The method consists of heating a substance with sulphuric acid, which decomposes the organic substance by oxidation to liberate the reduced nitrogen as ammonium sulphate. In this step potassium sulphate is added to increase the boiling point of the medium (from 337 °C to 373 °C) . Chemical decomposition of the sample is complete when the initially very dark-coloured medium has become clear and colourless.
The solution is then distilled with a small quantity of sodium hydroxide, which converts the ammonium salt to ammonia. The amount of ammonia present, and thus the amount of nitrogen present in the sample, is determined by back titration. The end of the condenser is dipped into a solution of boric acid. The ammonia reacts with the acid and the remainder of the acid is then titrated with a sodium carbonate solution by way of a methyl orange pH indicator.
- Degradation: Sample + H2SO4 → (NH4)2SO4(aq) + CO2(g) + SO2(g) + H2O(g)
- Liberation of ammonia: (NH4)2SO4(aq) + 2NaOH → Na2SO4(aq) + 2H2O(l) + 2NH3(g)
- Capture of ammonia: B(OH)3 + H2O + NH3 → NH4+ + B(OH)4−
- Back-titration: B(OH)3 + H2O + Na2CO3 → NaHCO3(aq) + NaB(OH)4(aq) + CO2(g) + H2O
The digested reaction mixture, on cooling, is transferred to a round bottomed distillation flask, and distilled with a concentrated alkali solution (NaOH). Ammonia produced is absorbed in a known volume of HCl solution of a known strength.


Let,
Mass of the organic compound = W g
Volume of the standard acid required for complete neutralization of the evolved ammonia = V mL
Normality of the standard solution of acid = N
From the law of equivalence (normality equation),
1000 mL of 1 N acid = 1000 mL of 1 N NH3 = 17g NH3 = 14 g nitrogen
Then,
V mL of N acid = V mL of NH3
NV milli equivalent of acid = NV milli equivalent of ammonia
Therefore,






This information is very helpful for me. It's very easy to understand and given in very simplified manner.. thank you
ReplyDeleteWe make the custom synthesis process more efficient and cost effective while maintaining the highest standards of quality and reliability. 1-butyl-2,3-dimethylimidazolium perchlorate
ReplyDeleteWrite the name of the element
ReplyDeleteWrite the formula of element
ReplyDelete