
For each reaction below, determine whether the primary reaction is SN1, SN2, E1, or E2, and then draw the product.
Note: Me = methyl (CH3)
problem set 1

SOLUTION
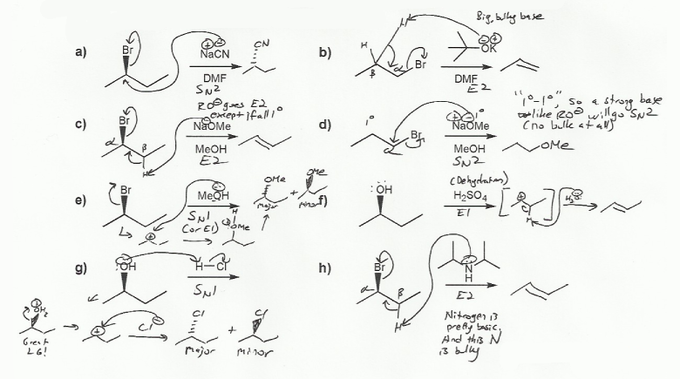
a) NaCN is charged! (Na+ and CN-), so it's SN2 or E2. CN is not a strong base, so it's SN2.
b) KOtBu (potassium tert-butoxide) is charged, so it's SN2 or E2. -OtBu is a strong base, so if anything is more bulky than 1º it will go E2. -OtBu is 3º, so it will definitely go E2 (KOtBu is a classic E2 reagent).
c) NaOMe is charged so E2 or SN2. NaOMe is a strong base, so if anything >1º it will go E2. -OMe is 1º (actually, not even 1º), but the alkyl halide is 2º, so it will go E2.
d) NaOMe is charged so E2 or SN2. NaOMe is a strong base, so if anything >1º it will go E2. But in this case there is no bulk whatsoever- nothing is >1º! NaOMe is 1 and the alkyl halide is also 1º, so it will go SN2.
e) Methanol (MeOH) is neutral so probably E1 or SN1. Methanol is a weak base and there's no bulk, so SN1. In general water and alcohol do a mixture of SN1 and E1 with alkyl halides (mostly SN1).
f) H2SO4 is acidic so probably E1 or SN1. Can't be SN1 though because there is no nucleophile in H2SO4. (HSO4- is a very weak nucleophile). An alcohol with H2SO4 or H3PO4 is a dehydration reaction- E1.
g) H2SO4 is acidic so probably E1 or SN1. In this case we have a nucleophile- Cl-, so it will go SN1.
h) Amines are neutral but they don't so SN1/E1- they tend to go SN2/E2, because they are basic (an amine solution has a basic pH). This amine is really bulky so it will go E2.
problem set 2
Rank the following compounds in order of decreasing reactivity with water (solvolysis). (1 = most reactive)

This is an SN1 reaction. We know this because none of the reagents have charges (H2O is neutral; if it were HO-, it would probably be SN2 or E2). So a carbocation will be formed in this reaction, and the compound whose carbocation is the most stable will react the fastest. So the 3º bromide will react fastest with water.

Rank the following compounds in order of decreasing reactivity with NaI in acetone. (1 = most reactive)

This is an SN2 reaction. We know this because NaI is an SN2 reagent- charges give it away; when we see charges (Na+ is positive and I- is negative) it's probably an SN2 reaction. So we want reactants that are less substituted: methyl is more reactive than 1º, which is more reactive than 2º, etc. So methyl bromide reacts the fastest with NaI.

Rank the following electrophiles in order of decreasing reactivity with NaN3 in DMF. (1 = most reactive)

Good leaving groups are stable. Larger ions tend to be more stable than smaller atoms (due to a smaller charge:size ratio. See problem 288), so when going down the periodic table, stability increases. I- is more stable than Br-, which is more stable than Cl-, etc. So I- is the best leaving group, and 2-iodobutane will react the fastest with a nucleophile.

Indicate the reagents necessary to carry out each transformation.

For a), the wedge remains a wedge, so we have to do two SN2 reactions (wedge to dash to wedge again). So we use PBr3 to turn the OH into a Br. (Bromination of an alcohol with PBr3 is an SN2 reaction and so inverts stereochemistry).
For b), the wedge becomes a dash, so we can only do one SN2 reaction. So instead of using PBr3 to make the OH a better leaving group, we use RSO2Cl, which doesn't break the carbon-oxygen bond and so doesn't invert the stereochemistry.

No comments:
Post a Comment