1. The E2 Reaction
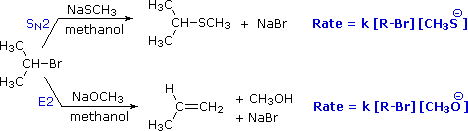
..............If two or more different of beta-hydrogens are present in a given reactant, then several constitutionally isomeric alkenes may be formed by an E2 elimination.
The transition states responsible for the above are
example :
example
In any case in an elimination reaction if a conjugated double bond is formed the it is more stable
example
2-bromobutane and 2-bromo-2,3-dimethylbutane elimination examples given below.
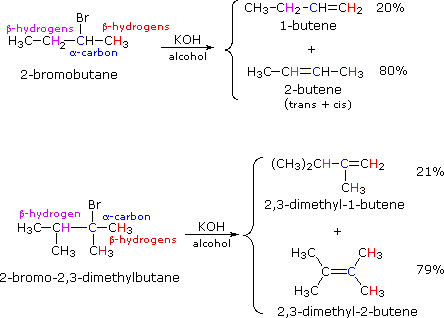
By using the strongly basic hydroxide nucleophile, we get elimination.
These results point to a strong regioselectivity favoring the more highly substituted product double bond, an empirical statement generally called the Zaitsev Rule.
These results point to a strong regioselectivity favoring the more highly substituted product double bond, an empirical statement generally called the Zaitsev Rule.
Zaitsev's rule (or Saytseff's rule) is an empirical rule for
predicting the favored alkene product(s) in elimination
Zaitsev stated, "The alkene formed in greatest amount is the one that corresponds to removal of the hydrogen from the β-carbon having the fewest hydrogen substituents."
Steric effects
In E2 elimination reactions, methoxide, orsodium ethoxide – is used for an E2 elimination, the Zaitsev product is typically favored over the least substituted alkene, known as the Hofmann product.
For example, treating 2-bromo-2-methylbutane with sodium ethoxide in ethanol produces the Zaitsev product with moderate selectivity.
Due to steric interactions, a bulky base – such as potassium t-butoxide, triethylamine, cannot readily abstract the proton that would lead to the Zaitsev product.
In these situations, a less sterically hindered proton is preferentially abstracted instead.
As a result, the Hofmann product is typically favored when using bulky bases.
When 2-bromo-2-methylbutane is treated with potassium t-butoxide instead of sodium ethoxide, the Hofmann product is favored.
as the bulkiness of the base increases ,it tends to attack at that beta hydrogen that is less stearically hindered
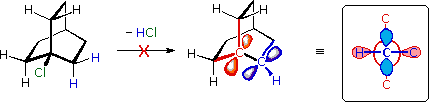
Bredt's Rule
For example, the bicyclooctyl 3º-chloride shown below , but it does not undergo elimination, even when treated with a strong base (e.g. KOH or KOC4H9).
There are six equivalent beta-hydrogens that might be attacked by base (two of these are colored blue as a reference), so an E2 reaction seems possible
. The problem with this elimination is that the resulting double bond would be constrained in a severely twisted (non-planar) configuration by the bridged structure of the carbon skeleton.
The carbon atoms of this twisted double-bond are colored red and blue respectively, and a Newman projection looking down the twisted bond is drawn on the right.
Because a pi-bond cannot be formed, the hypothetical alkene does not exist. Structural prohibitions such as this are often encountered in small bridged ring systems, and are referred to as Bredt's Rule.
There are six equivalent beta-hydrogens that might be attacked by base (two of these are colored blue as a reference), so an E2 reaction seems possible
. The problem with this elimination is that the resulting double bond would be constrained in a severely twisted (non-planar) configuration by the bridged structure of the carbon skeleton.
The carbon atoms of this twisted double-bond are colored red and blue respectively, and a Newman projection looking down the twisted bond is drawn on the right.
Because a pi-bond cannot be formed, the hypothetical alkene does not exist. Structural prohibitions such as this are often encountered in small bridged ring systems, and are referred to as Bredt's Rule.
Bredt's Rule should not be applied blindly to all bridged ring systems.
If large rings are present their conformational flexibility may permit good overlap of the p-orbitals of a double bond at a bridgehead.
DOUBLE BONDS AT BRIDGE HEADS IS NOT POSSIBLE .
This is similar to recognizing that trans-cycloalkenes cannot be prepared if the ring is small (3 to 7-membered), but can be isolated for larger ring systems.
Stereochemistry of the E2 Reaction
WE WILL LOOK IT UP IN THE NEXT POST















No comments:
Post a Comment