• In conjugated dienes, the π bonds are separated by exactly one single bond.
• In isolated dienes, the π bonds are separated by two or more single bonds.
Conjugated dienes represent a special category, because they contain one continuous system of overlapping p orbitals—that is, the two π bonds together comprise one functional group
composed of four overlapping p orbitals.
There fore , conjugated dienes exhibit special properties
and reactivity.
and 1,3-Butadiene is a conjugated diene
Electrophilic Addition
Addition of HX Across 1,3-Butadiene .
When butadiene is treated with HBr, electrophillic addition takes place,instead of one
two products are formed .
These two products can be explained with a two-step mechanism:
protonation to form a carbocation followed by nucleophilic attack. which is :
first electrophile attacks leading to formation of allylic carbocation that
undergoes rearrangement .These two carbocations attract the nucleophile that leeds to formation of the final product .
Thermodynamic Control vs. Kinetic Control
The expected addition product from reactions of this kind is the result of 1,2-addition, i.e. bonding to the adjacent
carbons of a double bond and the unexpected product
comes from 1,4-addition
Product compositions are temperature dependent, as the
addition of HBr to 1,3-butadiene demonstrates.
CH2=CH-CH=CH2 + HBr  reaction temperature | CH3CHBr-CH=CH2 + 1,2 addition yield | CH3CH=CHCH2Br 1,4 addition yield |
| 0 ºC 40 ºC | 70% 15% | 30% 85% |
Bonding of an electrophilic atom or group to one of the end
carbon atoms of a conjugated diene (#1) generates an allyl
cation intermediate.
Such cations are stabilized by charge delocalization, and it is
this delocalization that accounts for the 1,4-addition product
produced in such addition reactions.
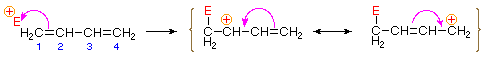
Nucleophillic attack to first acrbocation leads to 1,2 product.
Nucleophillic attack to second acrbocation leads to 1,4 product.
An explanation for the temperature influence is shown in the
following energy diagram for the addition of HBr to 1,3-
butadiene. 1,2 product. is termed kinetic product.. At higher
temperatures, equilibrium is established between the
products, and the thermodynamically favored 1,4 product.
dominates.1,4 product is termed thermodynamic product.
All said and done 1,2 product , termed kinetic product , requires lower activation energy and is formed at loewr temperatures as major product.1,4 product is the minor product.
At higher temperatures ,as per the requirements of
reaction, 1,4 product domminates ,since energy requirements in the form of higher activation energy is achieved
All said and done 1,2 product , termed kinetic product , requires lower activation energy and is formed at loewr temperatures as major product.1,4 product is the minor product.
At higher temperatures ,as per the requirements of
reaction, 1,4 product domminates ,since energy requirements in the form of higher activation energy is achieved








Sir,In the above mentioned 1,2 and1,4 addition since in case of conjugated systems the pi electrons are always delocalised among the p orbitals of the molecule, so, how is it certain that the electrophilic addition occurs only at the respective 1st and 2nd carbocation??
ReplyDeleteSir , even if the addition occurs suggested the way above can we describe such a reaction in case of of isolated systems??Is it possible to predict the exact product in case of isolated systems as it donot involve in delocalisation of pi eletrons??
ReplyDeleteas soon as proton attacks conjugated pi electron system ,attack takes place to give
ReplyDeletea secondary allylic carbocation .Hence delocalisation for all the 4 carbons is lost.How ever this secondary allylic carbocation can rearrange.Now Br- attacks both the carbocations to give 1,2 and 1,4 addition products.Both the probabilities exist.
Since in 1,4 addition more bond changes take place this leads to higher activation energy requirements.While 1,2 addition addition requires lower activation.
In isolated systems such carbocation rearrangements are non existant.Therefore
reactions are predominantly 1.12