AMINO ACIDS
Aside from playing an important role in protein and enzyme synthesis, amino acids are considered very crucial for your good health, since they contribute considerably to the health of the human nervous system, hormone production, and muscular structure.
In addition, they are needed for vital organs and cellular structure.
If a person experiences low levels of the essential amino acids, this may cause hormonal imbalances, lack of concentration, irritability, and even depression.
Properties
Amino acids are crystalline solids able to dissolve in water.
Meanwhile, they only dissolve sparingly in organic solvents, and the extent of their solubility depends on the size and nature of the side chain.
Amino acids feature very high melting points - up to 200-300°C, and other properties vary for each particular amino acid.
Classifications
Experts classify amino acids based on lots of different features.
One of them is whether or not people can acquire them through the diet.
According to this factor, scientists recognize 3 types:
the nonessential,
essential, and
conditionally essential amino acids.
However, the classification as essential or nonessential doesn't actually reflect their importance, as all twenty of them are necessary for human health.
Those 8 called essential (or indispensable) can't be produced by the body and therefore should be supplied by food: Leucine, Isoleucine, Lysine, Threonine, Methionine, Phenylalanine, Valine, and Tryptophan.
One more amino acid, Histidine, can be considered semi-essential, as the human body doesn't always need dietary sources of it.
Meanwhile, conditionally essential amino acids aren't usually required in the human diet, but are able to become essential under some circumstances.
Finally, nonessential ones are produced by the human body either out of the essential ones or from normal proteins breakdown.
These include Asparagine, Alanine, Arginine, Aspartic acid, Cysteine, Glutamic acid, Glutamine, Praline, Glycine, Tyrosine, and Serine.
One more classification depends on the side chain structure, and experts recognize 5 types in this classification:
1. containing sulfur (Cysteine and Methionine)
2. neutral (Asparagine, Serine, Threonine, and Glutamine)
3. acidic (Glutamic acid and Aspartic acid) and basic (Arginine and Lysine)
4. alphatic (these include Leucine, Isoleucine, Glycine, Valine, and Alanine)
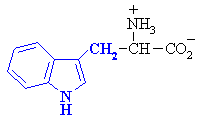
5. aromatic (these include Phenylalanine, Tryptophan, and Tyrosine)
Finally, there's another classification based on structure of the side chain that divides the list of twenty into 4 groups,
two of which are main groups and two are subgroups:
non-polar, polar, acidic and polar, basic and polar.
For example, side chains having pure hydrocarbon alkyl or aromatic groups are considered non-polar, and their list includes Phenylalanine, Glycine, Valine, Leucine, Alanine, Isoleucine, Proline, Methionine, and Tryptophan.

Meanwhile, if the side chain contains different polar groups like amides, acids, and alcohols, they are classified as polar.
Their list includes Tyrosine, Serine, Asparagine, Threonine, Glutamine, and Cysteine.
Further classification goes for acidic-polar (includes Aspartic Acid and Glutamic Acid),
if the side chain has a carboxylic acid, and basic-polar (includes Lysine, Arginine, and Histidine), if the side chain contains an amino group.
Finally, nonessential ones are produced by the human body either out of the essential ones or from normal proteins breakdown.
These include Asparagine, Alanine, Arginine, Aspartic acid, Cysteine, Glutamic acid, Glutamine, Praline, Glycine, Tyrosine, and Serine.
1. containing sulfur (Cysteine and Methionine)
2. neutral (Asparagine, Serine, Threonine, and Glutamine)
3. acidic (Glutamic acid and Aspartic acid) and basic (Arginine and Lysine)
4. alphatic (these include Leucine, Isoleucine, Glycine, Valine, and Alanine)
5. aromatic (these include Phenylalanine, Tryptophan, and Tyrosine)
Finally, there's another classification based on structure of the side chain that divides the list of twenty into 4 groups,
two of which are main groups and two are subgroups:
non-polar, polar, acidic and polar, basic and polar.

Amino acids with uncharged side chains
| POLAR SIDE CHAINS | NON POLAR SIDE CHAINS | ||
|---|---|---|---|
| SERINE |  | GLYCINE |  |
| THREONINE |  | ALANINE |  |
| TYROSINE |  | CYSTEINE (1) |  |
| ASPARAGINE |  | VALINE |  |
| GLUTAMINE |  | LEUCINE |  |
| ISOLEUCINE |  | ||
| PROLINE |  | ||
| METHIONINE |  | ||
| PHENYLALANINE |  | ||
| TRYPTOPHAN |  | ||
Amino acids with charged side chains
Charged side chains are POLAR.
| ACIDIC SIDE CHAINS | BASIC SIDE CHAINS | ||
|---|---|---|---|
| ASPARTIC ACID |  | LYSINE |  |
| GLUTAMIC ACID |  | ARGININE |  |
| HISTIDINE |  | ||
- (1) Paired cysteines allow disulfide bonds to form in proteins: -CH2-S-S-CH2-
α-Amino Acids
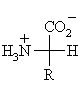
The generic structure of an α-amino acid
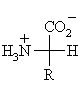
The generic structure of an α-amino acid
- The 20 naturally occurring α-amino acids used by cells to synthesise proteins can be generally represented by the generic formula shown above.
- The means the main difference between the various amino acids lies in the structure of the "R" group.
- These 20 α-amino acids can be sub-classified according to how the properties of other functional groups in the "R" group influence the system.
- non-polar side chains (e.g. alkyl groups)
- polar (e.g. amides, alcohols)
- acidic (e.g. carboxylic acids, phenols)
- basic (e.g. amines)
Amino acids with non-polar side chains. The hydrophobic side chains are chemically unreactive and tend to aggregate rather than be exposed to the aqueous environment, so they tend to found on the interior of proteins. Hydrophobic means "water hating" - remember "oil and water don't mix" and "like dissolves like" - this is because non-polar hydrocarbons do not interact with polar water molecules in an energetically favourable way - they would rather interact with another non-polar hydrocarbon molecule : this it the hydrophobic effect - the aggregation of non-polar systems in an aqueous environment.
(see also "micelles")
(see also "micelles")
Amino acids with polar side chains. These are side chains can be involved in hydrogen bonding interactions. Cysteine is important because of its ability to form disulfide bonds.
| Abbreviations | |||
 | |||
 | |||
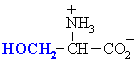 | |||
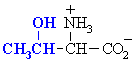 | |||
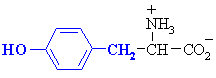 | |||
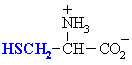 |
Amino acids with acidic side chains. These carboxylate group will be -ve at physiological pH. Often involved at the active sites of enzymes, in hydrogen bonding interactions and in acid/base type reactivity.
| Abbreviations | |||
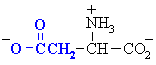 | |||
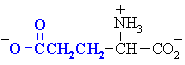 |
Amino acids with basic side chains. Often involved at the active sites of enzymes, in hydrogen bonding interactions and in acid/base type reactivity (e.g. histidine)
| Abbreviations | |||
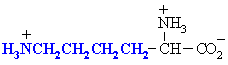 | |||
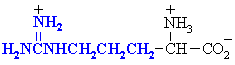 | |||
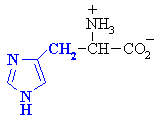 |
Isoelectronic point, pI
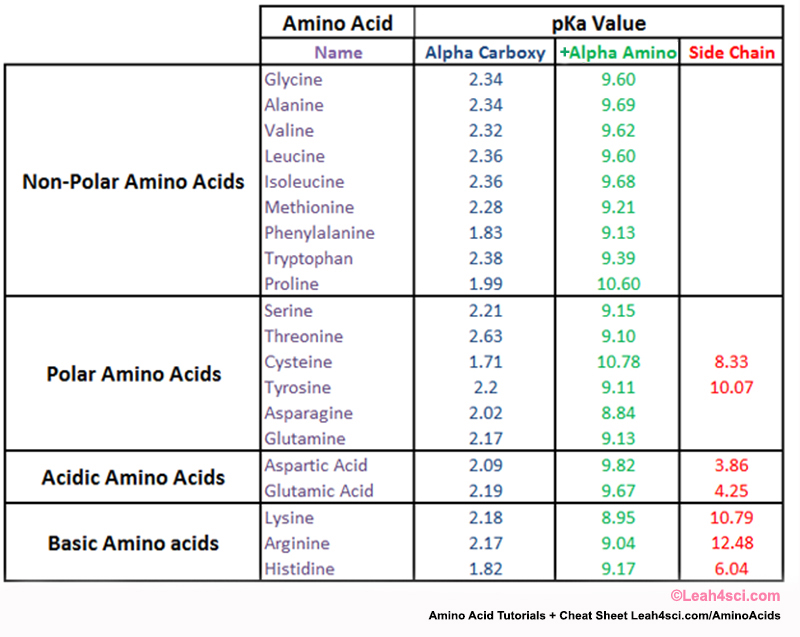 There are 3 cases to consider....
There are 3 cases to consider....
The isoelectronic point will be halfway between, or the average of, these two pKas, i.e. pI = 1/2 (pKa1 + pKa2).
This is most readily appreciated when you realise that at very acidic pH (below pKa1) the amino acid will have an overall +ve charge and at very basic pH (above pKa2 ) the amino acid will have an overall -ve charge.
For the simplest amino acid, glycine, pKa1= 2.34 and pKa2 = 9.6, pI = 5.97.
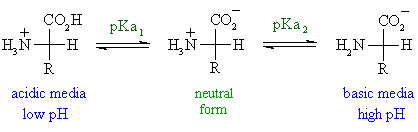
The other two cases introduce other ionisable groups in the side chain "R" described by a third acid dissociation constant, pKa3
- The isoelectronic point or isoionic point is the pH at which the amino acid does not migrate in an electric field.
- This means it is the pH at which the amino acid is neutral, i.e. the zwitterion form is dominant.
- A table of pKa and pI values can be found below .
- The pI is given by the average of the pKas that involve the zwitterion, i.e. that give the boundaries to its existence.

- neutral side chains
The isoelectronic point will be halfway between, or the average of, these two pKas, i.e. pI = 1/2 (pKa1 + pKa2).
This is most readily appreciated when you realise that at very acidic pH (below pKa1) the amino acid will have an overall +ve charge and at very basic pH (above pKa2 ) the amino acid will have an overall -ve charge.
For the simplest amino acid, glycine, pKa1= 2.34 and pKa2 = 9.6, pI = 5.97.

- acidic side chains
The pI will be at a lower pH because the acidic side chain introduces an "extra" negative charge.
So the neutral form exists under more acidic conditions when the extra -ve has been neutralised.
For example, for aspartic acid shown below, the neutral form is dominant between pH 1.88 and 3.65, pI is halfway between these two values, i.e. pI = 1/2 (pKa1 + pKa3), so pI = 2.77.
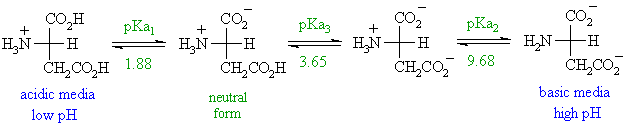
- basic side chains
The pI will be at a higher pH because the basic side chain introduces an "extra" positive charge.
So the neutral form exists under more basic conditions when the extra +ve has been neutralised.
For example, for histidine, , the neutral form is dominant between pH 6.00 and 9.17, pI is halfway between these two values, i.e. pI = 1/2 (pKa2 + pKa3), so pI = 7.59.
Table of pKa and pI values
- The pKa values and the isoelectronic point, pI, are given below for the 20 α-amino acids.
- pKa1= α-carboxyl group, pKa2 = α-ammonium ion, and pKa3 = side chain group.
| Amino acid | pKa1 | pKa2 | pKa3 | pI |
| Glycine | 2.34 | 9.60 | --- | 5.97 |
| Alanine | 2.34 | 9.69 | --- | 6.00 |
| Valine | 2.32 | 9.62 | --- | 5.96 |
| Leucine | 2.36 | 9.60 | --- | 5.98 |
| Isoleucine | 2.36 | 9.60 | --- | 6.02 |
| Methionine | 2.28 | 9.21 | --- | 5.74 |
| Proline | 1.99 | 10.60 | --- | 6.30 |
| Phenylalanine | 1.83 | 9.13 | --- | 5.48 |
| Tryptophan | 2.83 | 9.39 | --- | 5.89 |
| Asparagine | 2.02 | 8.80 | --- | 5.41 |
| Glutamine | 2.17 | 9.13 | --- | 5.65 |
| Serine | 2.21 | 9.15 | --- | 5.68 |
| Threonine | 2.09 | 9.10 | --- | 5.60 |
| Tyrosine | 2.20 | 9.11 | --- | 5.66 |
| Cysteine | 1.96 | 8.18 | --- | 5.07 |
| Aspartic acid | 1.88 | 9.60 | 3.65 | 2.77 |
| Glutamic acid | 2.19 | 9.67 | 4.25 | 3.22 |
| Lysine | 2.18 | 8.95 | 10.53 | 9.74 |
| Arginine | 2.17 | 9.04 | 12.48 | 10.76 |
| Histidine | 1.82 | 9.17 | 6.00 | 7.59 |
Titration Curve:
The titration curve for alanine, shown below, demonstrates this relationship.
At a pH lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge.
At a pH greater than 10, the amine exists as a neutral base and the carboxyl as its conjugate base, so the alanine molecule has a net negative charge.
At intermediate pH's the zwitterion concentration increases, and at a characteristic pH, called the isoelectric point (pI), the negatively and positively charged molecular species are present in equal concentration.
This behavior is general for simple (difunctional) amino acids. Starting from a fully protonated state, the pKa's of the acidic functions range from 1.8 to 2.4 for -CO2H, and 8.8 to 9.7 for -NH3(+).
The isoelectric points range from 5.5 to 6.2. Titration curves show the neutralization of these acids by added base, and the change in pH during the titration.
As noted earlier, the titration curves of simple amino acids display two inflection points, one due to the strongly acidic carboxyl group (pKa1 = 1.8 to 2.4), and the other for the less acidic ammonium function (pKa2 = 8.8 to 9.7). For the 2º-amino acid proline, pKa2 is 10.6, reflecting the greater basicity of 2º-amines.
Some amino acids have additional acidic or basic functions in their side chains.
These compounds are listed in the table on the right. A third pKa, representing the acidity or basicity of the extra function, is listed in the fourth column of the table.
The pI's of these amino acids (last column) are often very different from those noted above for the simpler members.
As expected, such compounds display three inflection points in their titration curves, illustrated by the titrations of arginine and aspartic acid shown below.
For each of these compounds four possible charged species are possible, one of which has no overall charge.
Formulas for these species are written to the right of the titration curves, together with the pH at which each is expected to predominate.
The very high pH required to remove the last acidic proton from arginine reflects the exceptionally high basicity of the guanidine moiety at the end of the side chain.
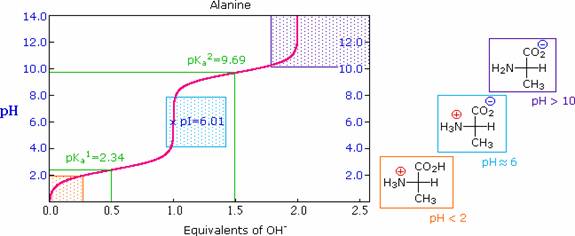
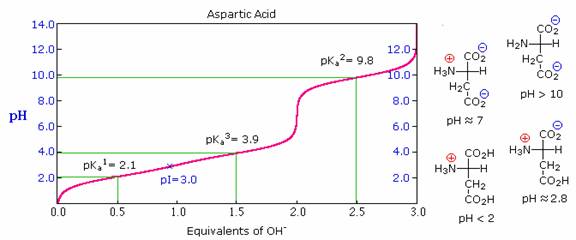
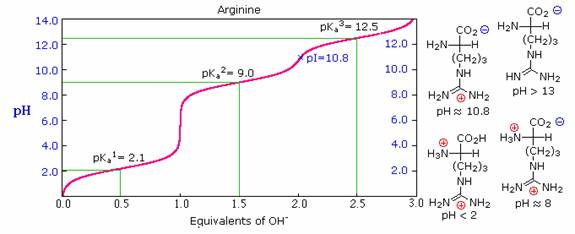

Calculation of pI for an Amino Acid.
To print this page, be sure that your web browser is set to full screen (click the right most arrow at the very top of the page). Then go to 'print preview' under 'file' and preview the printed page before you print it.The pI is the pH where the molecule exists in an uncharged state. Here are some practice problems for calculating the pI of an amino acid.
1. What is the pI of histidine?
Answer:
For histidine pKa1=2.3, pKa2=6.0 and pKa3=9.6.

At the pI the net charge of the molecule is zero. To find the pI, average the two pKa values on either side of the neutral form of histidine.
For histidine pKa1=2.3, pKa2=6.0 and pKa3=9.6.

At the pI the net charge of the molecule is zero. To find the pI, average the two pKa values on either side of the neutral form of histidine.
- (pKa2 + pKa3)/2 = pI
Amino Acid Stereochemistry
- Stereochemistry was introduced and most recently revisited for carbohydrates
- Here we will look at Fischer projections, the D-, L- notation of amino acids
- It's a good idea to review the basics of these topics if you do remember them before continuing.
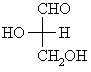
For the 20 α-amino acids that occur naturally in proteins, if we focus on the α-center, a chirality center, and draw the Fischer projection putting the -CO2H group up, then the ammonium group, NH3+, will be on the left, making it like L-glyceraldehyde where the -OH is on the left (review ?).
Hence, we have the L-amino acids.
alanine tryptophan S-(-)-glyceraldehyde
or
L-glyceraldehyde
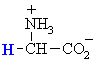
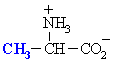
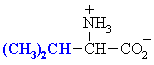
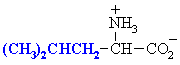
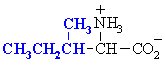
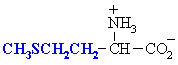
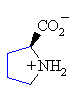
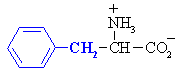
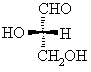
We make the custom synthesis process more efficient and cost effective while maintaining the highest standards of quality and reliability. Acid Red 87
ReplyDeleteA very informative post on Amino Acids.
ReplyDeleteAvail the most useful amino acids at: Amino Acid traders in India