Basicity of Amines, Acidity of Ammonium Ions
- N lone pair relatively easily protonated
- note that Kb x Ka = [H+] [OH-] = Kw = 10-14
or pKa + pKb = 14 - recall that when pH = pKa , there are equal concentrations of the conjugate acid and conjugate base present (i.e., RNH2 and RNH3+ )
- for typical aliphatic amines, pKb = 3 - 4
so pKa = 10 - 11 for their ammonium ions
so at around pH 10 - 11 , RNH2 and RNH3+ are both present - for typical aromatic amines, pKb = 9 - 10
so pKa = 4 - 5 for their ammonium ions
so at around pH 4 - 5 , ArNH2 and ArNH3+ are both present - water solubility of amines can be easily changed with pH
aromatic amines are water-soluble (protonated) below pH 4
aliphatic amines are water-soluble (protonated) below pH 9
Basicity Trends
Amines as Bases
|
• deciding the stronger of two acids, e.g. H-A1 and H-A2

• the stronger acid has the more stable anion
• the stronger acid corresponds to the more favorable "reaction", left to right
• deciding the stronger of two bases, e.g. B1 and B2

• there are no anions here, but the stronger base STILL corresponds to the more favorable reaction
Example

• measure basicity in terms of acidity (pKa) of conjugate acid (we will not use the pKb scale)

• compare (and don't confuse!!)

Trends in Basicity ??

• no clear trends among the aliphatic amines.....
But

• (minor) resonance delocalization (i.e. partial bonding) stabilizes (lowers energy) of the non-bonding electrons on N, these electrons are less reactive
• aromatic amines are thus less basic than aliphatic amines

• clear decrease in basicity with decreasing energy of non-bonding electrons as the hybridization of the orbitals gains more s character and less p character
• the protonated nitrile is about the strongest acid that we discuss this entire course
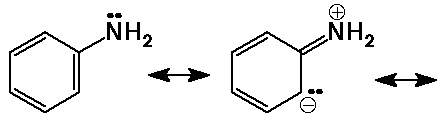
why aromatic amines are less basic ?
- aromatic amines are less basic due to resonance delocalization of the N lone pair
important conclusion : conjugation reduces basic nature .
- amides are nonbasic due to strong delocalization of the N lone pair
- electron withdrawing effects decrease basicity
because the N lone pair is less available for bonding to a proton
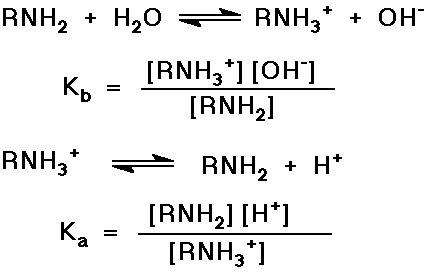
No comments:
Post a Comment