E2 ELEMINATION REACTIONS
E2 describes an elimination that has a bimolecular rate-determining step that must involve the base.
Loss of the leaving group is simultaneous with the removal of the proton by the base.
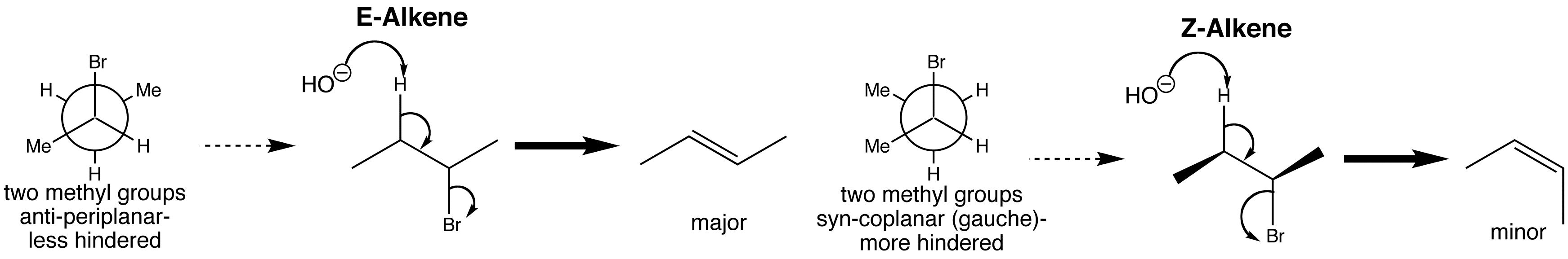
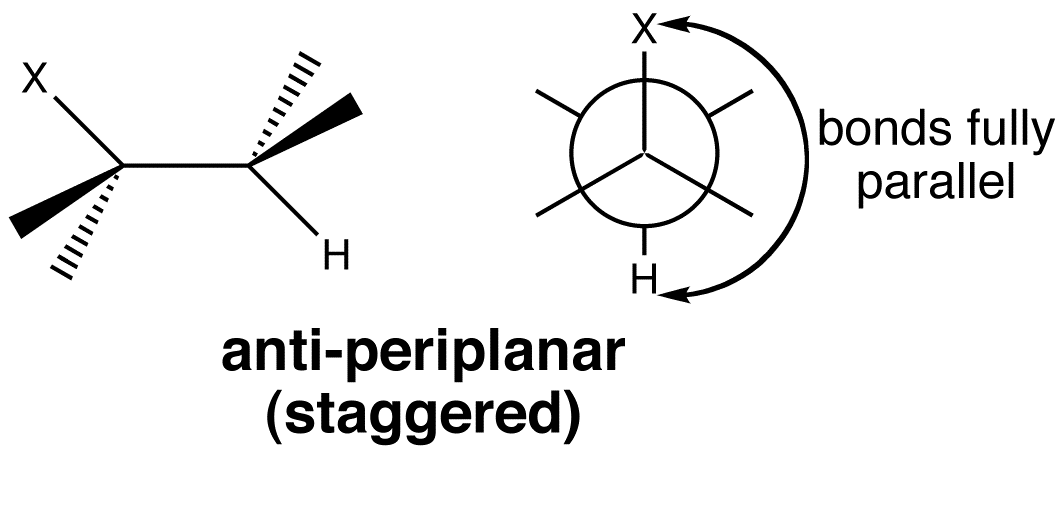
In an E2 eliminations, two orbitals have to lie in the same plane for best overlap.
E2 elimination takes place from the anti-periplanar conformation, as this is the most stable conformation due to its staggered nature.
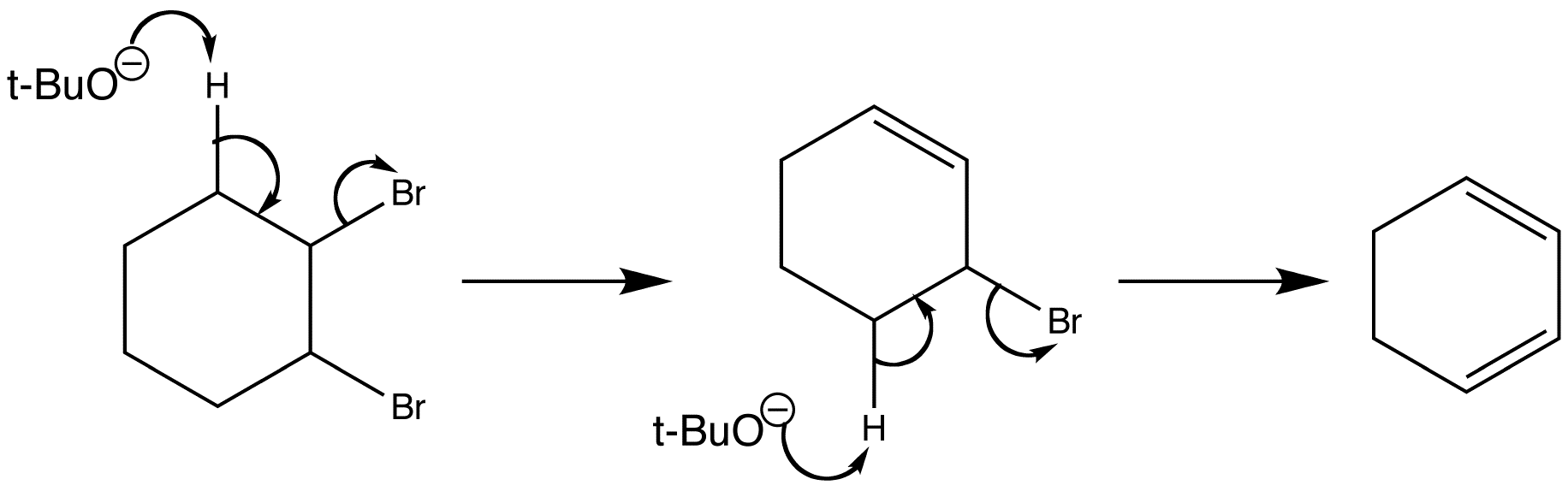
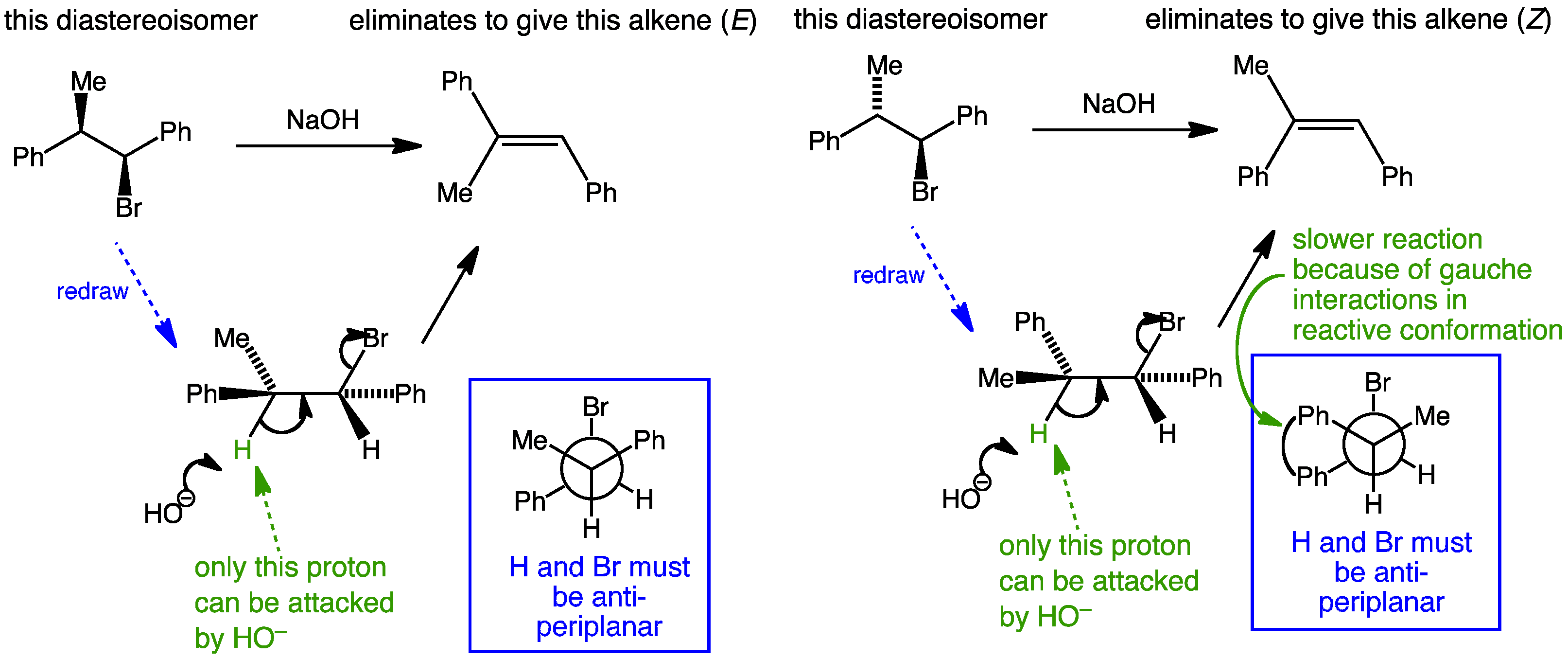
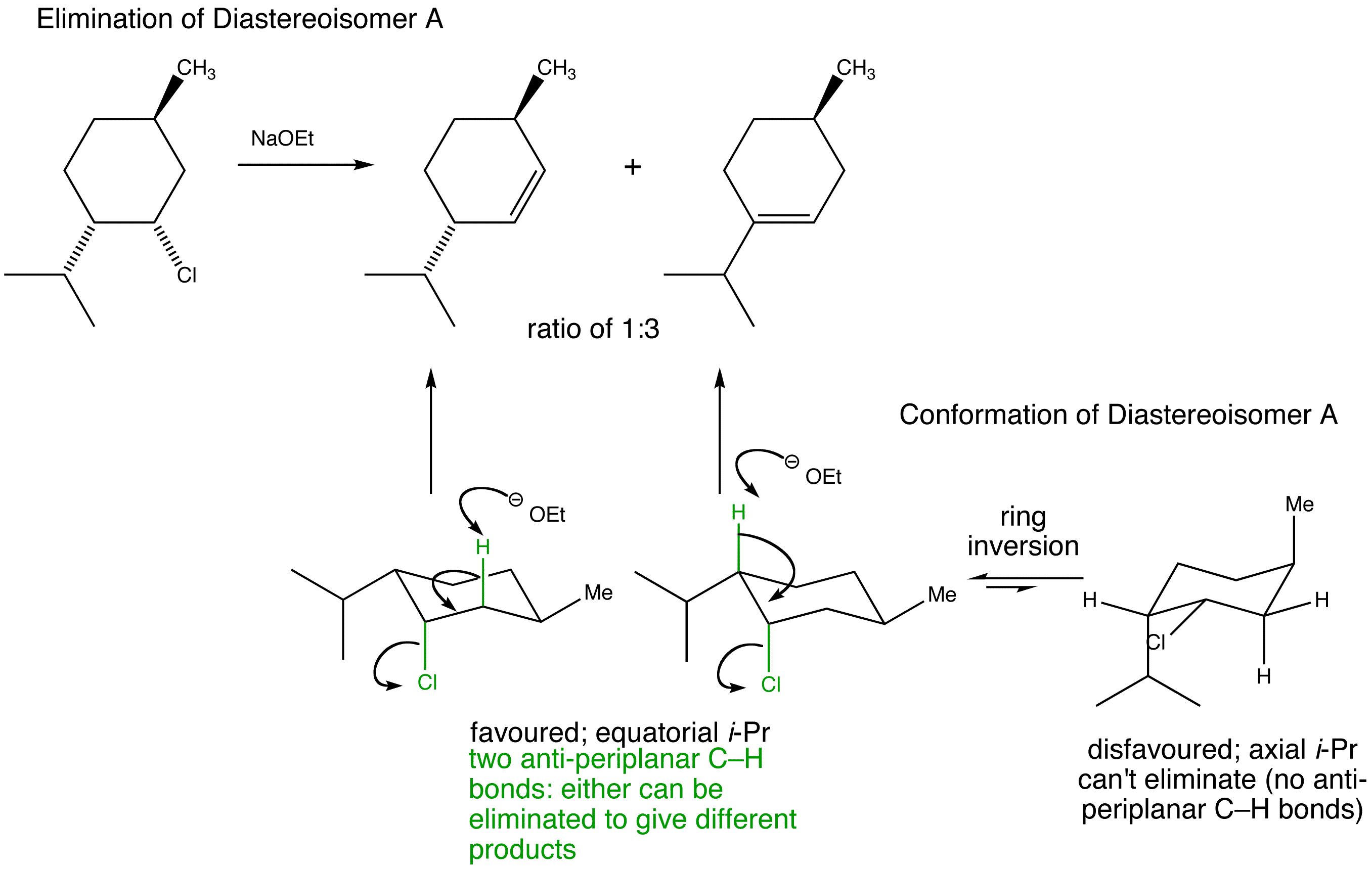
Stereospecific E2 eliminations - two diastereoisomeric chlorides Diastereoisomer A and Diastereoisomer Bderived from menthol give two different regioisomers of alkene.
Manipulate the conformations to explore the arrangements of the protons and the chlorine. Are they anti-periplanar as required for E2?
Manipulate the conformations to explore the arrangements of the protons and the chlorine. Are they anti-periplanar as required for E2?
Examine the animated energy plots of Diastereoisomer A and Diastereoisomer B to determine the relative energies of the anti-periplanar conformations required for E2 elimination.
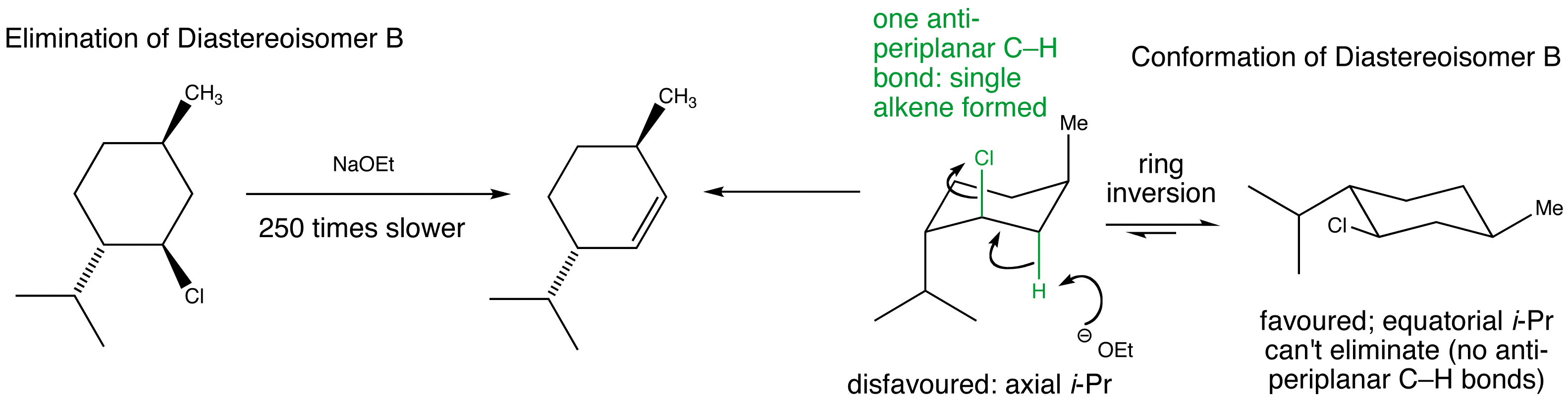
Stereospecific E2 eliminations - two diastereoisomeric chlorides derived from menthol give two different regioisomers of alkene.
E1 1ELIMINATION REACTIONS
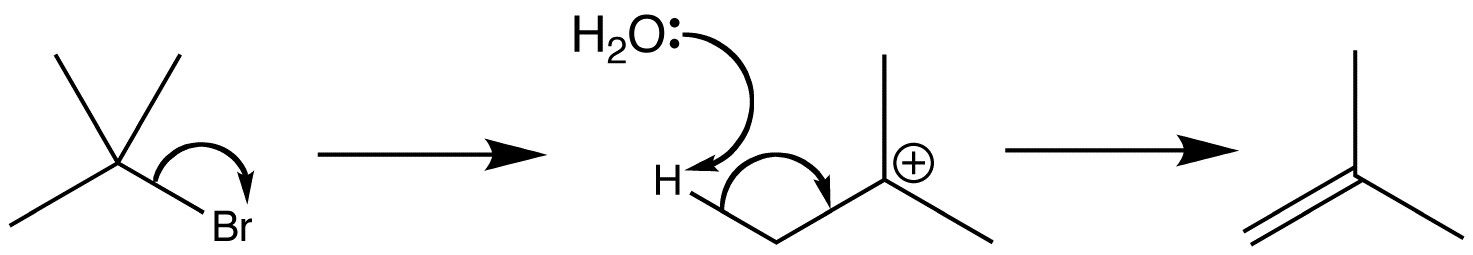
E1 describes an elimination reaction in which the rate-determining step is unimolecular and does not involve the base. The leaving group leaves in this step, and a proton is removed in a second step. This is an example of an E1 reaction which shows the formation of an alkene.
E1cb ELIMINATION REACTIONS
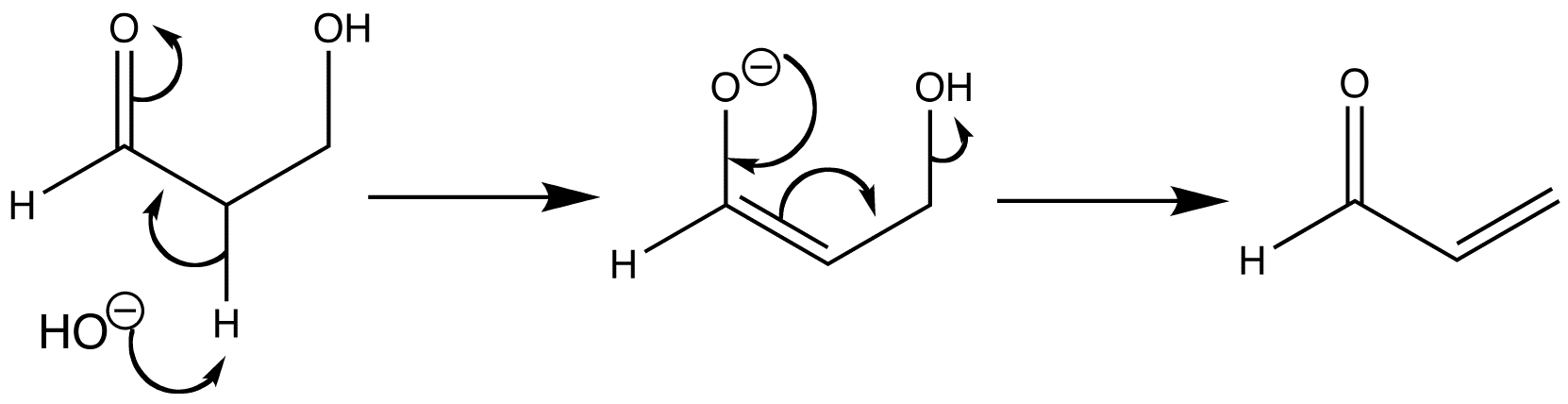
E1cB is an elimination reaction which looks similar to E2, only the leaving group can be a hydroxide, which cannot be the case in E2 elimination.
Negative charges are stabilized by conjugation with carbonyl groups.
The proton which is removed using a strong base is adjacent to a carbonyl group, which makes the proton rather acidic, and can therefore be removed by the base without the leaving group departing at the same time.
The resulting anion is stable due to delocalization on to the carbonyl group.
Although the anion is stabilized by the carbonyl group, it still prefers to lose a leaving group and become an alkene, which forms the rate-determining step for the elimination.
This is an example of an E1cB reaction which shows the formation of acrolein.
No comments:
Post a Comment