carbocation and Electrophilic Aromatic Substitution
Overall an electrophilic aromatic susbtitution (EArS) can be represented as follows:
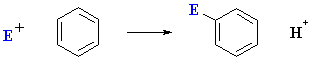
There are three fundamental components to an electrophilic aromatic substitution mechanism:
- formation of the new σ bond from a C=C in the arene nucleophile
- removal of the proton by breaking the C-H σ bond
- reforming the C=C to restore the aromaticity
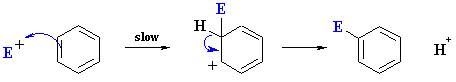 |
The mechanism is represented by the following series of events:
- Formation of the reactive electrophile, E+ (not shown here) from the reagents
- Slow reaction of the arene C=C with the E+ to give a resonance stabilised carbocation (see below)
- Loss of H+ from the carbocation to restore the C=C and the aromatic system
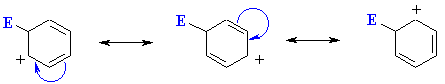
This carbocation is also described as the cyclohexdienyl cation or arenium ion or as a sigma-complex.
When phenol undergoes eas .carbo cation intermediate is formed which is stablized only at ortho and at para position.Not at meta position.

No comments:
Post a Comment