Formal Charges
Although formal charge can be calculated via a formula, see below, it is also possible to do it "instinctively" based on comparing structures. The "instinctive method" is quicker, and is probably what your instructor uses, but it does require more skill and an experience of common structures.
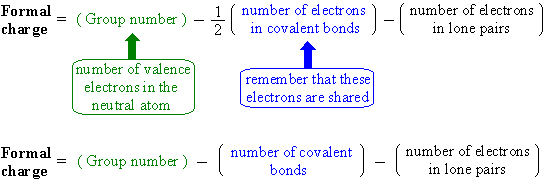
Formal charge equation is based on the comparing the number of electrons in the individual atom with that in the structure:
Instinctive method
This is based on comparing the structure with common, known neutral structures. To do this you need to recognise the common neutral structures: C 4 bonds, N 3 bonds, 1 lone pair, O 2 bonds, 2 lone pairs, F 1 bond, 3 lone pairs. Once mastered, this is much quicker.
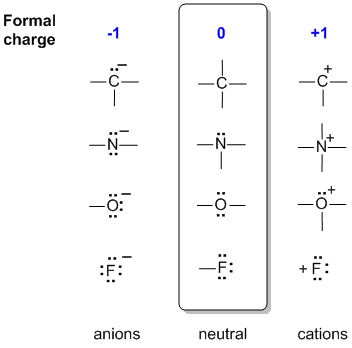
In the middle of the following diagram are the neutral bonding situations for C, N, O and a halogen, F. (Just think of each central atom bond being to a hydrogen).
To the left, a bond has been lost but converted to a lone pair, so the central atom has gained an electron and become -ve.
To the right, there are 2 scenarios:
- Not all atoms within a neutral molecule need be neutral
- The location of any charges is often important for understanding reactivity.
- Get into the habit of labelling the formal charges on any atoms.
- The formal charge on an atom can be calculated using an equation or by instinct (!)
- Check out some questions ?
Although formal charge can be calculated via a formula, see below, it is also possible to do it "instinctively" based on comparing structures. The "instinctive method" is quicker, and is probably what your instructor uses, but it does require more skill and an experience of common structures.
Formal charge equation is based on the comparing the number of electrons in the individual atom with that in the structure:

This is based on comparing the structure with common, known neutral structures. To do this you need to recognise the common neutral structures: C 4 bonds, N 3 bonds, 1 lone pair, O 2 bonds, 2 lone pairs, F 1 bond, 3 lone pairs. Once mastered, this is much quicker.
In the middle of the following diagram are the neutral bonding situations for C, N, O and a halogen, F. (Just think of each central atom bond being to a hydrogen).
To the left, a bond has been lost but converted to a lone pair, so the central atom has gained an electron and become -ve.
To the right, there are 2 scenarios:
- C and F have lost the shared electrons of a bond, so losing one electron from the "count" to become +ve.
- N and O have converted two unshared electrons into a shared pair of electrons in a bond, so losing one electron from the "count" to become +ve.
 |
No comments:
Post a Comment