Preparing Aldehydes and Ketones
Preparing Aldehydes
Partial oxidation of primary alcohols to aldehydes
This reaction uses pyridinium chlorochromate (PCC) in the absence of water (if water is present the alcohol will be oxidized further to a carboxylic acid).
in the absence of water (if water is present the alcohol will be oxidized further to a carboxylic acid). 
 in the absence of water (if water is present the alcohol will be oxidized further to a carboxylic acid).
in the absence of water (if water is present the alcohol will be oxidized further to a carboxylic acid). 

mechanism

from alcohols
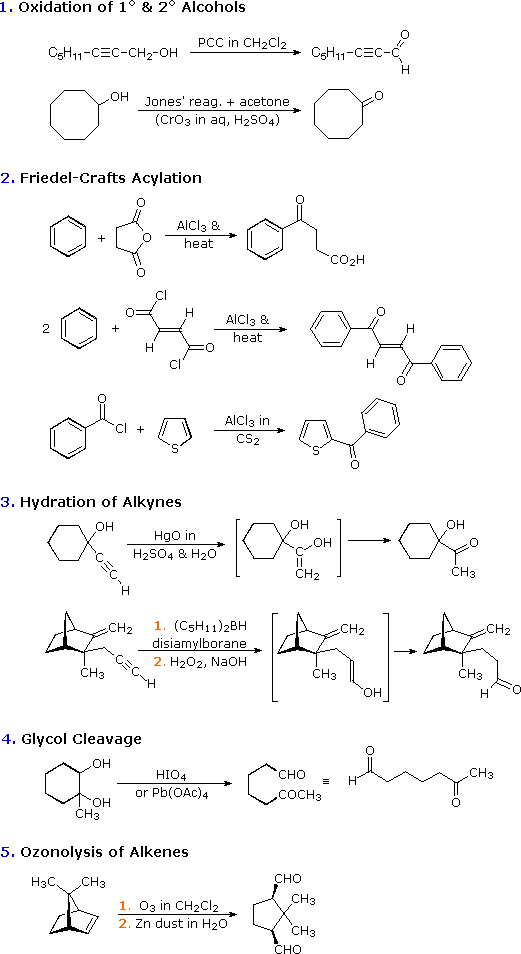
Swern oxidation


From fatty acids
(HCOO)2Ca + HEAT ----> HCHO + CaCO3 (CH3COO)2Ca + HEAT ----> acetone + CaCO3 (CH3COO)2Ca + (HCOO)2Ca ---->ethanaldehyde
Stephen reduction

mechanism

RCN + SnCl2+ HCL ----> RCH=NH2+Cl− ----> on hydrolysis gives RCHO
Here sulfur is used as a poisoner so that aldehyde formed doesn't get oxidised to the carboxylic acid. See the Wikipedia article for more detail.
Rosenmund reaction
RCOCl + Pd + BaSO4 + S ---->RCHO for solvent xylene is used
Preparing Ketones
From Grignard reagents
RCOOR' + R'MgX ---->RCOR + R'OH
From nitriles
MECHANISM FOR THE REACTION OF RMgX WITH A NITRILE
| |
| Step 1: The nucleophilic C in the organometallic reagent adds to theelectrophilic C in the polar nitrile group. Electrons from theC≡N move to the electronegative N creating an intermediate imine salt complex. | 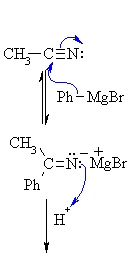 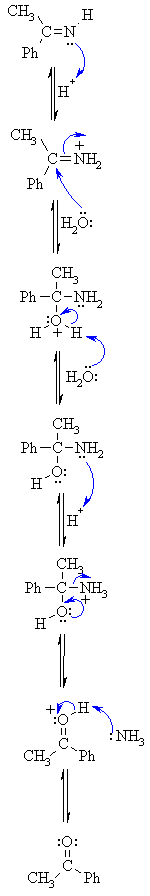 |
| Step 2: An acid/base reaction. On addition of aqueous acid, the intermediate salt protonates giving the imine. | |
| Step 3: An acid/base reaction. Imines undergo nucleophilic addition, but require activation by protonation (i.e. acid catalysis). | |
| Step 4: Now the nucleophilic O of a water molecule attacks the electrophilicCwith the π bond breaking to neutralise the change on the N. | |
| Step 5: An acid/base reaction. Deprotonate the O from the water molecule to neutralise the positive charge. | |
| Step 6: An acid/base reaction. Before the N system leaves, it needs to be made into a better leaving group by protonation. | |
| Step 7: Use the electrons on the O in order to push out the N leaving group, a neutral molecule of ammonia. | |
| Step 8: An acid/base reaction. Deprotonation reveals the carbonyl group ofthe ketone product. | |
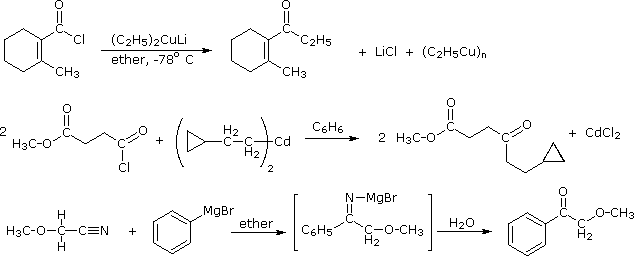
RCN + R'MgX ----> RCOR'(after hydrolysis) HCN does not react with RMgX as HCN has acidic hydrogen which results in RH being formed.
From gem dihalides
RCCl2R + strong base ----> RCOR
Oppenaur oxidation

mechanism :

The aluminium-catalyzed hydride shift from the α-carbon of an alcohol component to the carbonyl carbon of a second component, which proceeds over a six-membered transition state, is named Meerwein-Ponndorf-Verley-Reduction (MPV) or Oppenauer Oxidation (OPP) depending on the isolated product. If aldehydes or ketones are the desired products, the reaction is viewed as the Oppenauer Oxidation.
Non-enolizable ketones with a relatively low reduction potential, such as benzophenone, can serve as the carbonyl component used as the hydride acceptor in this oxidation.
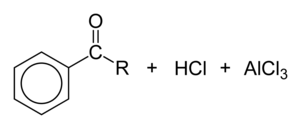
Friedel-Crafts acylation of aromatic compounds

Reaction mechanism
In a simple mechanistic view, the first step consists of dissociation of a chloride ion to form an acyl cation (acylium ion)
An aromatic ring reacts with a carboxylic acid chlorine (RCOCl) in the presence of AlCl3 to form an aryl ketone of the form ArCOR.
Oxidation of secondary alcohols to ketones
A secondary alcohol can be oxidised into a ketone using acidified potassium dichromate(VI) and heating under reflux.
The orange dichromate(VI) ion, Cr2O72-, is reduced to the green Cr3+(aq) ion.
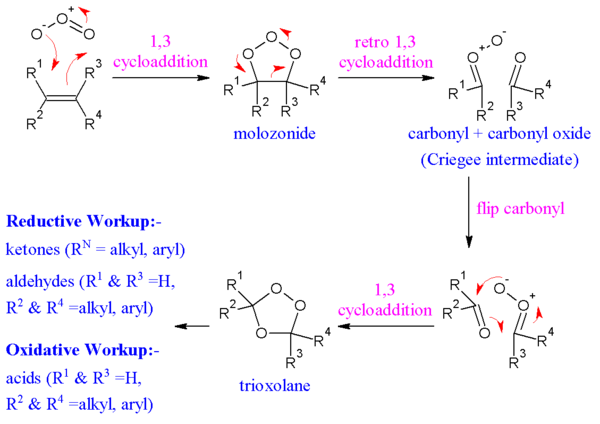
Ozonolysis of alkenes
It is a reaction in which the double bond is completely broken and the alkene molecule converted into two smaller molecules.
Ozonolysis (cleavage "by ozone) is carried out in two stages: first, addition of ozone to the double bond to form an ozonide ; and second, hydrolysis of the ozonide to yield the cleavage products.
Ozone gas is passed into a solution of the alkene in some inert solvent like carbon tetrachloride; evaporation of the solvent leaves the ozonide as a viscous oil. This unstable, explosive compound is not purified, but is treated directly with water, generally in the presence of a reducing agent. If oxidsing reagent is used, aldehyde or ketone if oxidisable can further oxidise into carboxylic acid which is not the case with reducing agents
In the cleavage products a doubly-bonded oxygen is found attached to each of the originally doubly-bonded carbons.
The function of the reducing agent, which is frequently zinc dust, is to prevent formation of hydrogen peroxide, which would otherwise react with the aldehydes and ketones. (Aldehydes, RCHO, are often converted into acids, RCOOH, for ease of isolation.)
Mechanism[edit]
The alkene and ozone form an intermediate molozonide in a 1,3-dipolar cycloaddition. Next, the molozonide reverts to its corresponding carbonyl oxide (also called the Criegee intermediate or Criegee zwitterion) and aldehyde or ketone in a retro-1,3-dipolar cycloaddition. The oxide and aldehyde or ketone react again in a 1,3-dipolar cycloaddition or produce a relatively stable ozonide intermediate (a trioxolane)
Hydration of alkynes
Water is added to an alkyne in a strong acid. The strong acid used is sulfuric acid and mercuric acid.









No comments:
Post a Comment