Baeyer-Villiger oxidation
Also known as: Baeyer-Villiger rearrangement

The Baeyer-Villiger oxidation is an organic reaction used to convert a ketone to an ester using a peroxyacid (such as mCPBA). The reaction of the ketone with the acid results in a tetrahedral intermediate, with an alkyl migration following to release a carboxylic acid. The more electron rich R group migrates to the oxygen in this concerted process, allowing for accurate prediction of the stereochemistry of the product.

Mechanism
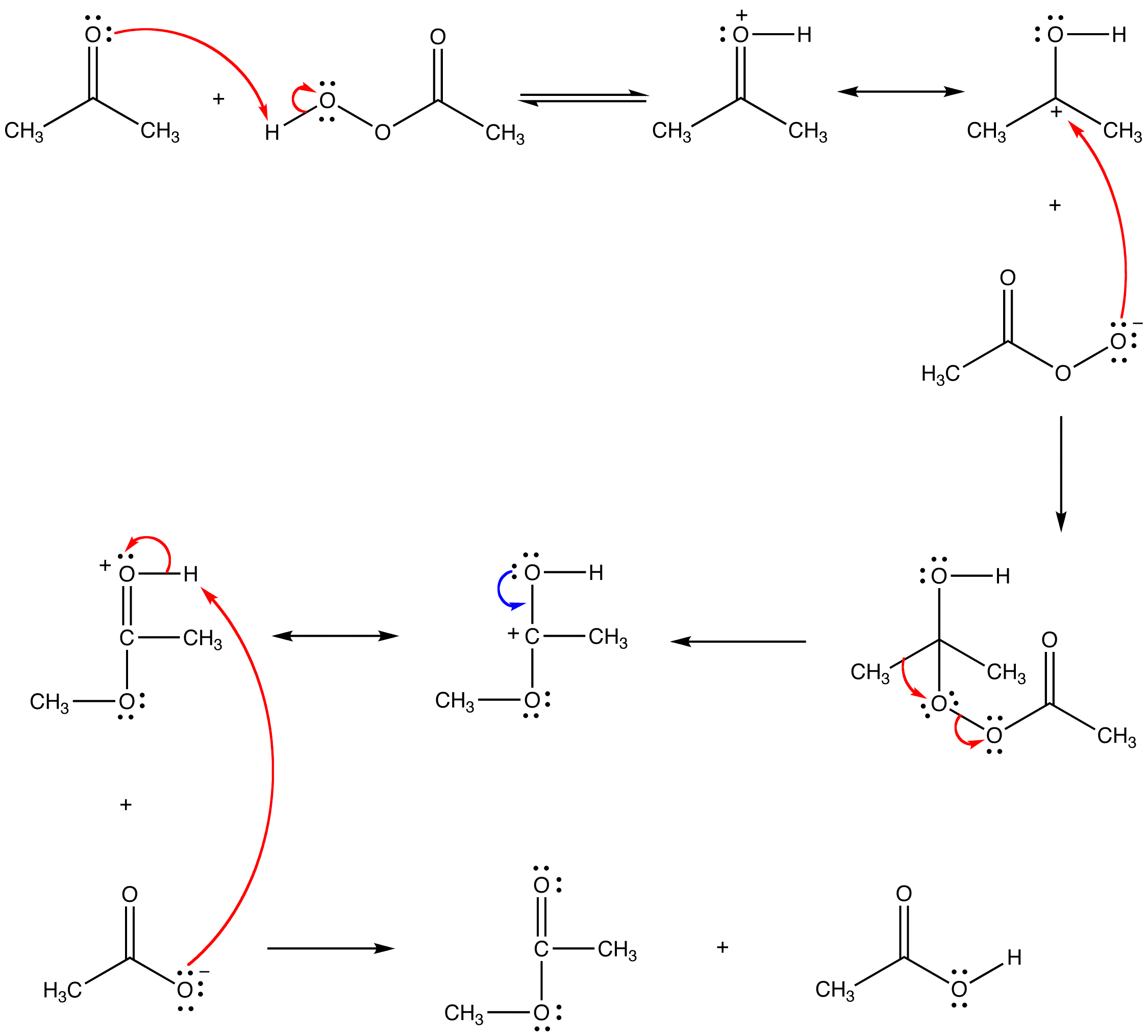
When the two ligands on the carbonyl carbon in the ketone are different, Baeyer-Villiger oxidation is regioselective. Of the two alpha carbons in the ketone, the one that can stabilize a positive charge more effectively, which is the more highly substituted one, migrates from carbon to oxygen preferentially.
Baeyer-Villiger Migratory Aptitude
the approximate order of decreasing ease of migration is hydrogen > tertiary alkyl > secondary alkyl > phenyl > primary alkyl > methyl
eg. 1:

eg. 2:
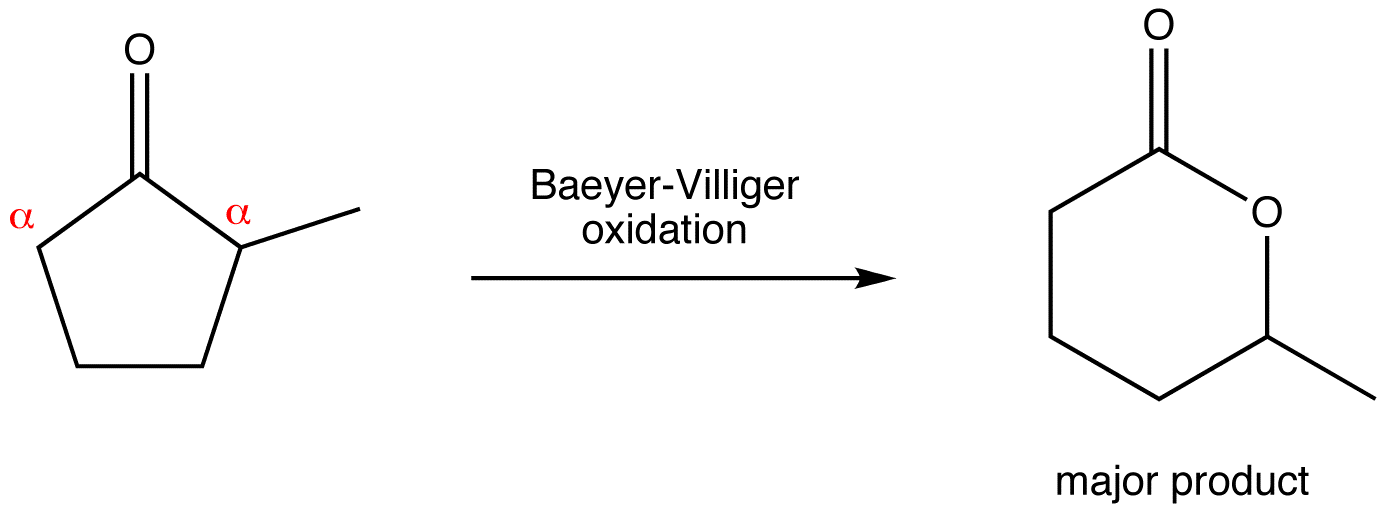

Baeyer-Villiger Migratory Aptitude
the approximate order of decreasing ease of migration is hydrogen > tertiary alkyl > secondary alkyl > phenyl > primary alkyl > methyl
No comments:
Post a Comment