Addition of 1o Amines to Form Enamines
Introduction
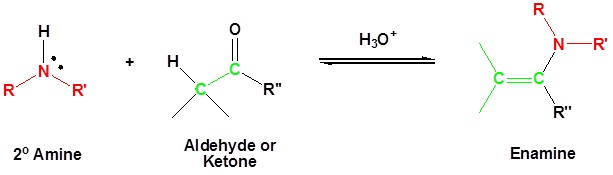
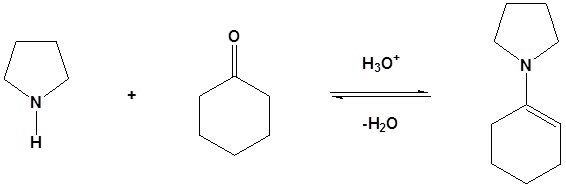
Most aldehydes and ketones react with 2º-amines to give products known as enamines. It should be noted that, like acetal formation, these are acid-catalyzed reversible reactions in which water is lost. Consequently, enamines are easily converted back to their carbonyl precursors by acid-catalyzed hydrolysis.
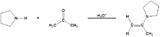
| Example |
Mechanism
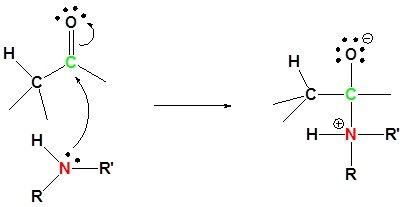
1) Nuleophilic attack
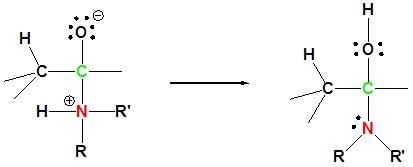
2) Proton transfer
3) Protonation of OH
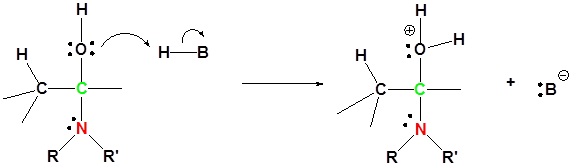
4) Removal of water
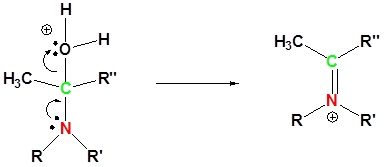
5) Deprotonation
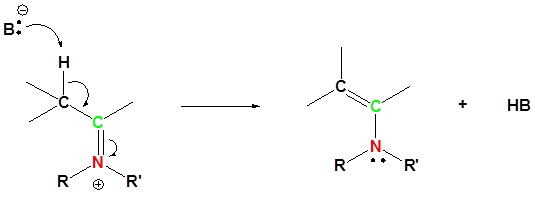
Alkylation of an Enamine
Enamined undergo an SN2 reaction with reactive alkyl halides to give the iminium salt. The iminium salt can be hydrolyzed back into the carbonyl.
Individual steps
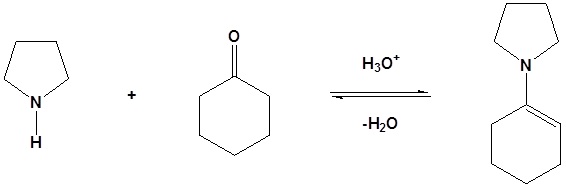
1) Formation of an enamine
2) SN2 Alkylation
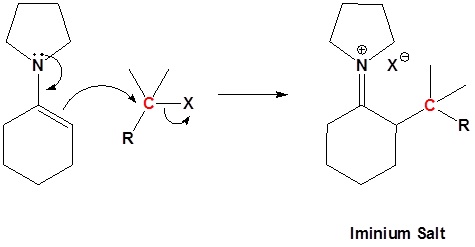 3) Reform the carbonyl by hydrolysis
3) Reform the carbonyl by hydrolysis
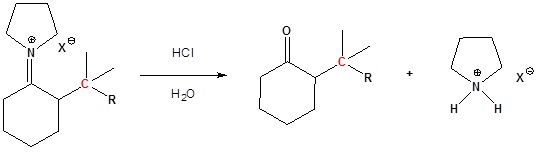
All three steps together:
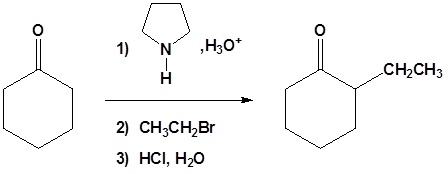
Enamined undergo an SN2 reaction with reactive alkyl halides to give the iminium salt. The iminium salt can be hydrolyzed back into the carbonyl.
Individual steps
1) Formation of an enamine

2) SN2 Alkylation

3) Reform the carbonyl by hydrolysis

All three steps together:

Acylation of Enamines
Enamine can react with acid halides to form β-dicarbonyls
1) Formation of the enamine
 2) Nucleophilic attack
2) Nucleophilic attack
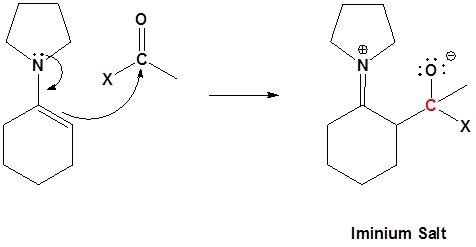 3) Leaving group removal
3) Leaving group removal
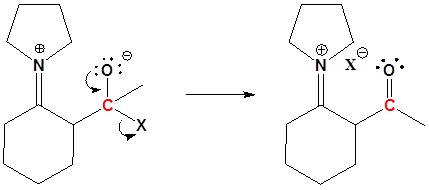 4) Reform the carbonyl by hydrolysis
4) Reform the carbonyl by hydrolysis
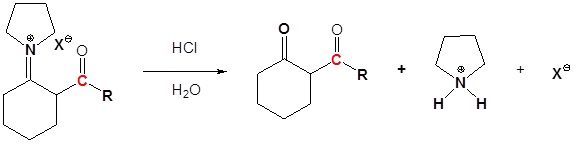
All three steps together:
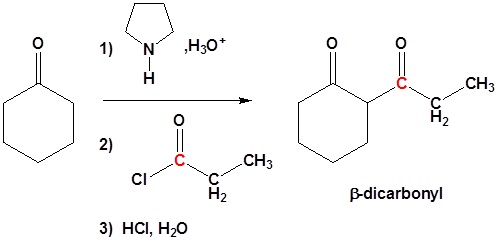
Enamine can react with acid halides to form β-dicarbonyls
1) Formation of the enamine

2) Nucleophilic attack

3) Leaving group removal

4) Reform the carbonyl by hydrolysis

All three steps together:

nett reaction
.png)
No comments:
Post a Comment