Addition of Water to form Hydrates (Gem-Diols)
It has been demonstrated that water, in the presence of an acid or a base, adds rapidly to the carbonyl function of aldehydes and ketones establishing a reversible equilibrium with a hydrate(geminal-diol or gem-diol). The word germinal or gem comes from the Latin word for twin,geminus.
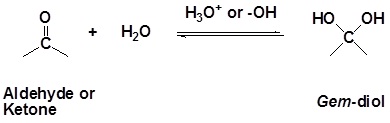
| Example 1 |
|---|
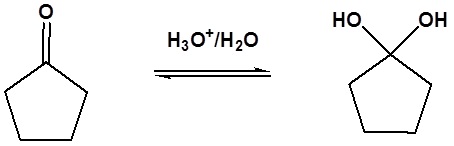 |
Going from Reactants to Products Simplified
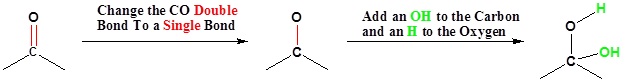
Reversibility of the Reaction
Isolation of gem-diols is difficult because the reaction is reversibly. Removal of the water during a reaction can cause the conversion of a gem-diol back to the corresponding carbonyl.
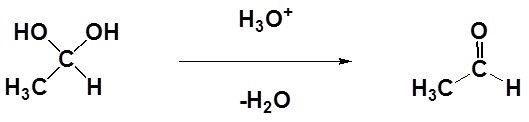
Factors Affecting the Gem-diol Equilibrium
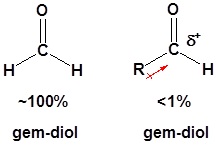
In most cases the resulting gem-diol is unstable relative to the reactants and cannot be isolated.
Exceptions to this rule exist, one being formaldehyde where the weaker pi-component of the carbonyl double bond, relative to other aldehydes or ketones, and the small size of the hydrogen substituents favor addition.
Thus, a solution of formaldehyde in water (formalin) is almost exclusively the hydrate, or polymers of the hydrate.
The addition of electron donating alkyl groups stabilized the partial positive charge on the carbonyl carbon and decreases the amount of gem-diol product at equilibrium.
Because of this ketones tend to form less than 1% of the hydrate at equilibrium.
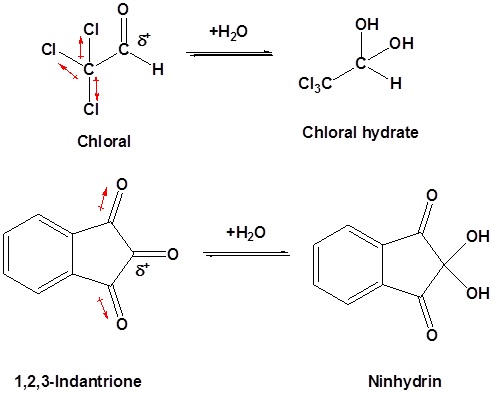
Likewise, the addition of strong electron-withdrawing groups destabilizes the carbonyl and tends to form stable gem-diols. Two examples of this are chloral, and 1,2,3-indantrione.
It should be noted that chloral hydrate is a sedative and has been added to alcoholic beverages to make a “Knock-out” drink also called a Mickey Finn. Also, ninhydrin is commonly used by forensic investigators to resolve finger prints.
Mechanism of Gem-diol Formation
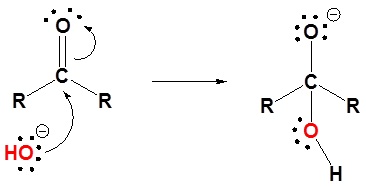
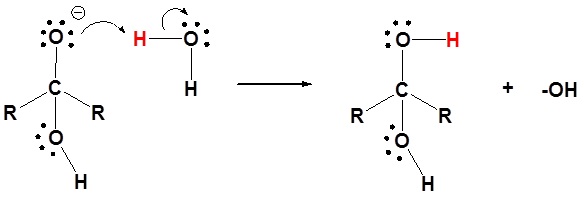
The mechanism is catalyzed by the addition of an acid or base. Note! This may speed up the reaction but is has not effect on the equilibriums discussed above. Basic conditions speed up the reaction because hydroxide is a better nucleophilic than water. Acidic conditions speed up the reaction because the protonated carbonyl is more electrophilic.
Basic conditions
1) Nucleophilic attack by hydroxide
2) Protonation of the alkoxide
Acidic conditions
1) Protonation of the carbonyl

2) Nucleophilic attack by water
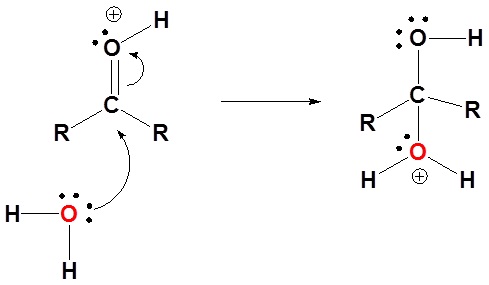
3) Deprotonation
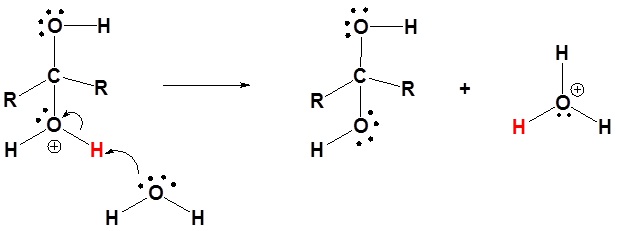
Problems
1) Draw the expected products of the following reactions.
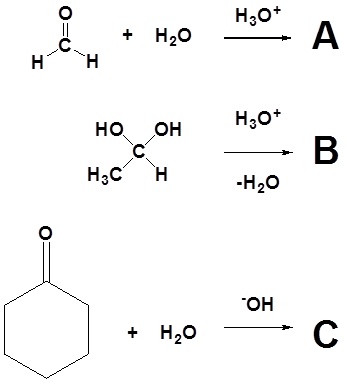
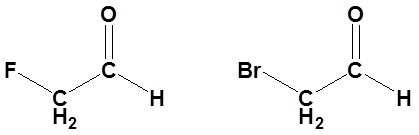
2) Of the following pairs of molecules which would you expect to form a larger percentage of gem-diol at equilibrium? Please explain your answer.
3) Would you expect the following molecule to form appreciable amount of gem-diol in water? Please explain your answer.
![13]a.jpg](http://chemwiki.ucdavis.edu/@api/deki/files/13120/13%5Da.jpg?revision=1)
Answers
1)
2) The compound on the left would. Fluorine is more electronegative than bromine and would remove more electron density from the carbonyl carbon. This would destabilize the carbonyl allowing for more gem-diol to form.
3) Although ketones tend to not form gem-diols this compound exists almost entirely in the gem-diol form when placed in water. Ketones tend to not form gem-diols because of the stabilizing effect of the electron donating alkyl group. However, in this case the electron donating effects of alkyl group is dominated by the presence of six highly electronegative fluorines.
No comments:
Post a Comment